Immunoprotective Effect of Coptis chinensis-Supplemented Diet on Streptococcus agalactiae Infection in Tilapia
Abstract
:1. Introduction
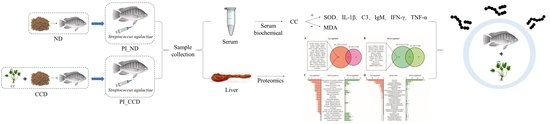
2. Materials and Methods
2.1. Experimental Fish and Pathogenic Bacteria
2.2. Experimental Feed
2.3. Experimental Stage and Grouping
2.4. Bacterial Culture and Inoculation Experiments
2.5. Sample Collection
2.6. Biochemical and Immunological Index Determination
2.7. iTRAQ Differential Proteomics Assay
2.8. Database Search and Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of CC on Biochemical Indexes in the Liver of Healthy and SA-Infected Tilapia
3.2. Effect of CC on the Serum Immune Indexes of Healthy and SA-Infected Tilapia
3.3. Protein Expression in SA-Infected Tilapia before and after CC Treatment
3.4. Changes in Protein Expression in CC-Treated Healthy Tilapia
3.5. Changes in Protein Expression in CC-Treated SA-Infected Tilapia
3.6. Functional Analysis of CC-Specific Regulatory Proteins in Tilapia before and after SA Infection
4. Discussion
4.1. CC Supplementation in Feed Enhances the Antioxidant Function of Tilapia
4.2. CC Supplementation in Feed Enhances the Immune Function of Tilapia
4.3. CC Regulates the Expression of Antimicrobial Proteins in the Liver of Tilapia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z. Research Advances on Tilapia Streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Barkham, T.; Zadoks, R.N.; Azmai, M.N.A.; Baker, S.; Bich, V.T.N.; Chalker, V.; Chau, M.L.; Dance, D.; Deepak, R.N.; van Doorn, H.R.; et al. One hypervirulent clone, sequence type 283, accounts for a large proportion of invasive Streptococcus agalactiae isolated from humans and diseased tilapia in Southeast Asia. PLOS Negl. Trop. Dis. 2019, 13, e0007421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, L.; Lou, G.; Zeng, H.-R.; Hu, J.; Huang, Q.; Peng, W.; Yang, X.-B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.Q.; Liu, D.L. Progress in pharmacological research of Coptis chinensis. Gansu Agric. 2019, 10, 97–99. [Google Scholar]
- Yu, F.X.; Cao, M.X.; Liang, W.W.; Wang, C.H.; Wang, S.; Wei, Y.Y.; Hu, T.J. Clinical application of Sanhuanglian powder in prevention of Streptococcicosis in tilapia. Anim. Husb. Feed Sci. 2020, 41, 86–91. [Google Scholar]
- Wang, C.H.; Yu, F.X.; Zhao, Y.; Liang, W.W.; Wei, Y.Y.; Hu, T.J. Clinical application effect of Sanhuanglian mixture in preventing tilapia against Streptococcosis. Guangxi J. Anim. Husb. Vet. Med. 2020, 36, 147–150. [Google Scholar]
- Niu, Z.W.; Fan, H.L.; Long, S.; Huang, J.; Zhu, H.F.; Huang, G.C.; Liang, J.Z. A Meta-analysis: The effect of three kinds of Chinese herbalmedicines for tilapia Streptococcus agalactiae. J. Fish. Res. 2016, 38, 409–414. [Google Scholar]
- Matumoto, M. A note on some points of calculation method of LD50 by Reed and Muench. Jpn. J. Exp. Med. 1949, 20, 175–179. [Google Scholar]
- Choi, W.; Lam, C.; Mo, W.; Cheng, Z.; Mak, N.; Bian, Z.; Wong, M. Effects of the modified Huanglian Jiedu decoction on the disease resistance in grey mullet (Mugil cephalus) to Lactococcus garvieae. Mar. Pollut. Bull. 2014, 85, 816–823. [Google Scholar] [CrossRef]
- Ji, D.W.; Li, M.Y.; Wang, T.Z.; Zhang, C.N.; Xu, Z.; Xu, W.T. Effects of low temperature stress periods on serum biochemical indexes in large yellow croaker Pseudosciaena crocea. Fish. Sci. 2009, 28, 1–4. [Google Scholar]
- Xu, X.-J.; Xu, B.; Wang, J.; Su, Y.-Q.; Zhang, Z.-W.; Chen, X. Studies on blood chemistry indices and histopathology of Pseudosciaena crocea artificially challenged with Vibrio harveyi. J. Fish. China 2010, 34, 618–625. [Google Scholar] [CrossRef]
- Mu, D.; Jiang, Y.; He, J.; Zhang, Y.; Yang, D.; Liu, Q.; Wang, Z. Dietary supplementation of propolis enhanced the innate immune response against Edwardsiella piscicida challenge in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2022, 124, 273–279. [Google Scholar] [CrossRef]
- Ao, Q.W.; Lu, Y.J.; Lu, M.; Zhu, J.J. Effects of Streptococcus agalactiae infection on blood and hepatopancreatic tissue biochemical indices in different species of tilapia. Prog. Fish. Sci. 2020, 41, 167–173. [Google Scholar]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of interleukin-1β. Cytokine Growth Factor Rev. 2002, 13, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Danilko, K.V.; Korytina, G.F.; Akhmidishina, L.Z.; Ianbaeva, D.G.; Zagidullin, S.Z.; Victorova, T.V. Association of cytokines genes (IL1B, IL1RN, TNFA, LTA, IL6, IL8, and IL10) polymorphic markers with chronic obstructive pulmonary disease. Mol. Biol. 2007, 41, 26–36. [Google Scholar] [CrossRef]
- Roberts-Thomson, I.C.; Fon, J.; Uylaki, W.; Cummins, A.G.; Barry, S. Cells, cytokines and inflammatory bowel disease: A clinical perspective. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L. Insights into the biology and therapeutic implications of TNF and regulatory T cells. Nat. Rev. Rheumatol. 2021, 17, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.G.; Ma, Y.W.; Liu, F.; Meng, X.J. Effects of yam extract on growth performance and non-specific immune parameters of rainbow trout (Oncorhynchus mykiss). J. Henan Agric. Sci. 2020, 49, 167–172. [Google Scholar] [CrossRef]
- Castro, R.; Bromage, E.; Abós, B.; Pignatelli, J.; Granja, A.G.; Luque, A.; Tafalla, C. CCR7 Is Mainly Expressed in Teleost Gills, Where It Defines an IgD+IgM− B Lymphocyte Subset. J. Immunol. 2014, 192, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; La Patra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef]
- Li, X.T.; Ke, X.L.; Lu, M.X.; Liu, Z.G.; Wang, M.; Pang, J.C. Cloning and tissues expression analysis of complement C3 of tilapia. Biotechnology 2016, 26, 205–212. [Google Scholar] [CrossRef]
- Kui, L.L.; Liu, Y. Effects of Edwardsiella ictaluri inactivated vaccine on immune gene wxpression and activities of CAT, SOD and complement C3 in yellow catfish Pelteobagrus fulvidraco. Fish. Sci. 2015, 34, 150–154. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Achen, M.G.; Stacker, S.A. Lymphatic endothelium: An important interactive surface for malignant cells. Pulm. Pharmacol. Ther. 2006, 19, 51–60. [Google Scholar] [CrossRef]
- Bilodeau-Bourgeois, L.; Bosworth, B.G.; Peterson, B.C. Differences in mortality, growth, lysozyme, and Toll-like receptor gene expression among genetic groups of catfish exposed to virulent Edwardsiella ictaluri. Fish Shellfish Immunol. 2008, 24, 82–89. [Google Scholar] [CrossRef]
- Small, B.C.; Bilodeau, A.L. Effects of cortisol and stress on channel catfish (Ictalurus punctatus) pathogen susceptibility and lysozyme activity following exposure to Edwardsiella ictaluri. Gen. Comp. Endocrinol. 2005, 142, 256–262. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef]
- Zhuo, L.-C.; Chen, C.-F.; Lin, Y.-H. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2021, 117, 248–252. [Google Scholar] [CrossRef]
- Gobec, S. Inhibitors of Cathepsin B. Curr. Med. Chem. 2006, 13, 2309–2327. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Chen, Y.; Zhu, R.; Dong, C.; Li, Y.; Zhang, Q.; Gui, J. Expressional induction of Paralichthys olivaceus cathepsin B gene in response to virus, poly I:C and lipopolysaccharide. Fish Shellfish Immunol. 2008, 25, 542–549. [Google Scholar] [CrossRef]
- Li, C.; Song, L.; Tan, F.; Su, B.; Zhang, D.; Zhao, H.; Peatman, E. Identification and mucosal expression analysis of cathepsin B in channel catfish (Ictalurus punctatus) following bacterial challenge. Fish Shellfish Immunol. 2015, 47, 751–757. [Google Scholar] [CrossRef]
- Li, L.; Shi, Y.; Wang, R.; Huang, T.; Liang, W.; Luo, H.; Gan, X.; Huang, W.; Li, J.; Lei, A.; et al. Proteomic analysis of tilapia Oreochromis niloticus Streptococcus agalactiae strains with different genotypes and serotypes. J. Fish Biol. 2015, 86, 615–636. [Google Scholar] [CrossRef]
- Mazon-Moya, M.J.; Willis, A.R.; Torraca, V.; Boucontet, L.; Shenoy, A.R.; Colucci-Guyon, E.; Mostowy, S. Septins restrict inflammation and protect zebrafish larvae from Shigella infection. PLOS Pathog. 2017, 13, e1006467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Li, Y.; Yang, Y.; Li, C.; Yao, J.; Zeng, Q.; Qin, Z.; Liu, S.; Li, D.; Liu, Z. Septin genes in channel catfish (Ictalurus punctatus) and their involvement in disease defense responses. Fish Shellfish Immunol. 2016, 49, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chhottaray, C.; Das Mahapatra, K.; Saha, J.N.; Baranski, M.; Robinson, N.; Sahoo, P.K. Analysis of immune-related ESTs and differential expression analysis of few important genes in lines of rohu (Labeo rohita) selected for resistance and susceptibility to Aeromonas hydrophila infection. Mol. Biol. Rep. 2014, 41, 7361–7371. [Google Scholar] [CrossRef] [PubMed]
- Klupp, F.; Kahlert, C.; Franz, C.; Halama, N.; Schleussner, N.; Wirsik, N.M.; Warth, A.; Schmidt, T.; Ulrich, A.B. Granulin: An Invasive and Survival-Determining Marker in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 6436. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Lin, H.-J.; Lin, W.-F.; Wu, J.-L.; Gong, H.-Y. A potent tilapia secreted granulin peptide enhances the survival of transgenic zebrafish infected by Vibrio vulnificus via modulation of innate immunity. Fish Shellfish Immunol. 2018, 75, 74–90. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chou, H.-Y.; Liu, P.-C.; Wu, J.-L.; Gong, H.-Y. Granulin peptide GRN-41 of Mozambique tilapia is a novel antimicrobial peptide against Vibrio species. Biochem. Biophys. Res. Commun. 2019, 515, 706–711. [Google Scholar] [CrossRef]
- Saleh, M.; Kumar, G.; Abdel-Baki, A.-A.S.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Quantitative proteomic profiling of immune responses to Ichthyophthirius multifiliis in common carp skin mucus. Fish Shellfish Immunol. 2019, 84, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shinomiya, H.; Kirikae, T.; Hirata, H.; Asano, Y. Characterization of Murine Grancalcin Specifically Expressed in Leukocytes and Its Possible Role in Host Defense against Bacterial Infection. Biosci. Biotechnol. Biochem. 2004, 68, 894–902. [Google Scholar] [CrossRef] [PubMed]






| Material | ND | CCD |
|---|---|---|
| Ingredient (%) | ||
| Fish meal | 8.5 | 8.5 |
| Soybean meal | 40 | 40 |
| Rape seed meal | 20 | 20 |
| Wheat middlings | 13 | 13 |
| Calcium phosphate | 1.5 | 1.5 |
| Choline Chloride | 1.5 | 1.5 |
| Mineral premix a | 0.5 | 0.5 |
| Vitamin premix a | 0.5 | 0.5 |
| Microcrystalline cellulose | 9 | 9 |
| NaCl | 0.5 | 0.5 |
| Soybean oil | 2 | 2 |
| Carboxymethyl cellulose | 3 | 2 |
| Coptis chinensis powder | 0 | 1 |
| Total | 100 | 100 |
| Proximate composition (%) b | ||
| Crude protein | 32.90 | 32.94 |
| Crude lipid | 3.81 | 3.82 |
| Crude fiber | 16.90 | 15.96 |
| Crude ash | 6.40 | 6.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Yu, K.; Huang, K.; Lin, Q.; Liu, T. Immunoprotective Effect of Coptis chinensis-Supplemented Diet on Streptococcus agalactiae Infection in Tilapia. Fishes 2023, 8, 370. https://doi.org/10.3390/fishes8070370
Guo R, Yu K, Huang K, Lin Q, Liu T. Immunoprotective Effect of Coptis chinensis-Supplemented Diet on Streptococcus agalactiae Infection in Tilapia. Fishes. 2023; 8(7):370. https://doi.org/10.3390/fishes8070370
Chicago/Turabian StyleGuo, Ruijie, Kai Yu, Kai Huang, Qiang Lin, and Ting Liu. 2023. "Immunoprotective Effect of Coptis chinensis-Supplemented Diet on Streptococcus agalactiae Infection in Tilapia" Fishes 8, no. 7: 370. https://doi.org/10.3390/fishes8070370
APA StyleGuo, R., Yu, K., Huang, K., Lin, Q., & Liu, T. (2023). Immunoprotective Effect of Coptis chinensis-Supplemented Diet on Streptococcus agalactiae Infection in Tilapia. Fishes, 8(7), 370. https://doi.org/10.3390/fishes8070370