An Overview of the Pathogenesis and Treatment of Elbow Osteoarthritis
Abstract
:1. Introduction
2. Background and Pathogenesis
2.1. Primary Elbow Osteoarthritis
2.2. Post-Traumatic Osteoarthritis
2.3. Elbow Osteoarthritis in the Athlete
3. Clinical and Radiological Evaluation
3.1. Patient Evaluation
3.2. Rating Systems for Evaluation of the Elbow
3.3. Imaging
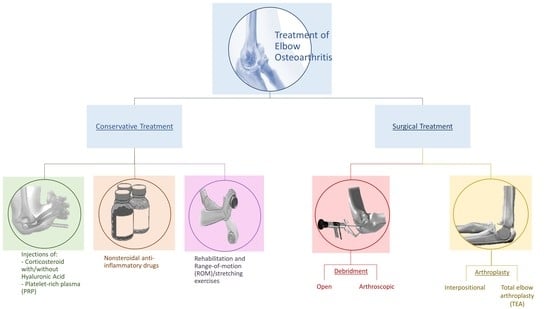
4. Treatment
4.1. Conservative Treatment
4.2. Surgical Treatment
4.3. Alternative and New Approaches
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Musumeci, G.; Loreto, C.; Imbesi, R.; Trovato, F.M.; Di Giunta, A.; Lombardo, C.; Castorina, S.; Castrogiovanni, P. Advantages of exercise in rehabilitation, treatment and prevention of altered morphological features in knee osteoarthritis. A narrative review. Histol. Histopathol. 2014, 29, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.H.; Rat, A.C.; Sellam, J.; Michel, M.; Eschard, J.P.; Guillemin, F.; Jolly, D.; Fautrel, B. Economic impact of lower-limb osteoarthritis worldwide: A systematic review of cost-of-illness studies. Osteoarthr. Cartil. 2016, 24, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. The Effect of Mechanical Loading on Articular Cartilage. J. Funct. Morphol. Kinesiol. 2016, 1, 154. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef]
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Méd. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Musumeci, G. Which is the Best Physical Treatment for Osteoarthritis? J. Funct. Morphol. Kinesiol. 2016, 1, 54. [Google Scholar] [CrossRef]
- Chammas, M. Post-traumatic osteoarthritis of the elbow. Orthop. Traumatol. 2014, 100, S15–S24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijink, A.; Vanhees, M.; van den Ende, K.; van den Bekerom, M.P.; van Riet, R.P.; Van Dijk, C.N.; Eygendaal, D. Biomechanical considerations in the pathogenesis of osteoarthritis of the elbow. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2313–2318. [Google Scholar] [CrossRef]
- Cheung, E.V.; Adams, R.; Morrey, B.F. Primary osteoarthritis of the elbow: Current treatment options. J. Am. Acad. Orthop. Surg. 2008, 16, 77–87. [Google Scholar] [CrossRef]
- Papatheodorou, L.K.; Baratz, M.E.; Sotereanos, D.G. Elbow arthritis: Current concepts. J. Hand Surg. 2013, 38, 605–613. [Google Scholar] [CrossRef]
- Gramstad, G. Management of Elbow Osteoarthritis. J. Bone Jt. Surg. 2006, 88, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Kingdom, U. Prevalence and etiology of symptomatic elbow osteoarthritis. J. Shoulder Elb. Surg. 1994, 3, 386–389. [Google Scholar] [CrossRef]
- Spector, T.D.; MacGregor, A.J. Risk factors for osteoarthritis: Genetics. Osteoarthr. Cartil. 2004, 12, S39–S44. [Google Scholar] [CrossRef] [PubMed]
- Yucesoy, B.; Charles, L.E.; Baker, B.; Burchfiel, C.M. Occupational and genetic risk factors for osteoarthritis: A review. Work 2015, 50, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loughlin, J. Genetic contribution to osteoarthritis development: Current state of evidence. Curr. Opin. Rheumatol. 2015, 27, 284–288. [Google Scholar] [CrossRef]
- Scanzello, C.R. Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Kokkalis, Z.T.; Schmidt, C.C.; Sotereanos, D.G. Elbow arthritis: Current concepts. J. Hand Surg. 2009, 34, 761–768. [Google Scholar] [CrossRef]
- Soojian, M.G.; Kwon, Y.W. Elbow arthritis. Bull. NYU Hosp. Joint Dis. 2007, 65, 61–71. [Google Scholar]
- McAuliffe, J.A. Surgical alternatives for elbow arthritis in the young adult. Hand Clin. 2002, 18, 99–111. [Google Scholar] [CrossRef]
- O’Driscoll, S.W. Elbow Arthritis: Treatment Options. J. Am. Acad. Orthop. Surg. 1993, 1, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, R.W.; Cohen, M.S. Primary Osteoarthritis and Posttraumatic Arthritis of the Elbow. Hand Clin. 2011, 27, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Fleisig, G.S.; Andrews, J.R.; Dillman, C.J.; Escamilla, R.F. Kinetics of baseball pitching with implications about injury mechanisms. Am. J. Sports Med. 1995, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Dietz, J. Osteoarthritis in Other Joints (Hip, Elbow, Foot, Ankle, Toes, Wrist) after Sports Injuries. Clin. Sports Med. 2005, 24, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; Hansmann, H.J.; Brocai, D.R.; Loew, M. Long Term Changes of the Throwing Arm of Former Elite Javelin Throwers. Int. J. Sports Med. 2001, 22, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Muroi, S.; Sugaya, H.; Takahashi, N.; Matsuki, K.; Kawai, N.; Osaki, M. Osteochondritis Dissecans of the Humeral Capitellum in Young Athletes: Comparison Between Baseball Players and Gymnasts. Orthopaedic J. Sports Med. 2017, 5, 2325967117692513. [Google Scholar] [CrossRef]
- Lee, J.; Steinmann, S.P. Elbow Arthritis in the Athlete. Oper. Tech. Sports Med. 2017, 25, 288–294. [Google Scholar] [CrossRef]
- McFarland, E.G.; Gill, H.S.; Laporte, D.M.; Streiff, M. Miscellaneous conditions about the elbow in athletes. Clin. Sports Med. 2004, 23, 743–763. [Google Scholar] [CrossRef]
- McLaughlin, R.E.; Savoie, F.H.; Field, L.D.; Ramsey, J.R. Arthroscopic treatment of the arthritic elbow due to primary radiocapitellar arthritis. Arthroscopy 2006, 22, 63–69. [Google Scholar] [CrossRef]
- Kelly, E.W.; Bryce, R.; Coghlan, J.; Bell, S. Arthroscopic debridement without radial head excision of the osteoarthritic elbow. Arthroscopy 2007, 23, 151–156. [Google Scholar] [CrossRef]
- Longo, U.G.; Franceschi, F.; Loppini, M.; Maffulli, N.; Denaro, V. Rating systems for evaluation of the elbow. Br. Med. Bull. 2008, 87, 131–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewald, F.C.; Scheinberg, R.D.; Poss, R.; Thomas, W.H.; Scott, R.D.; Sledge, C.B. Capitellocondylar total elbow arthroplasty. J. Bone Joint Surg. 1980, 62, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Broberg, M.A.; Morrey, B.F. Results of delayed excision of the radial head after fracture. J. Bone Joint Surg. 1986, 68, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Figgie, M.P.; Inglis, A.E.; Mow, C.S.; Figgie, H.E. Total elbow arthroplasty for complete ankylosis of the elbow. J. Bone Joint Surg. 1989, 71, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Turchin, D.C.; Beaton, D.E.; Richards, R.R. Validity of observer-based aggregate scoring systems as descriptors of elbow pain, function, and disability. J. Bone Joint Surg. 1998, 80, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- King, G.J.; Richards, R.R.; Zuckerman, J.D.; Blasier, R.; Dillman, C.; Friedman, R.J.; Gartsman, G.M.; Iannotti, J.P.; Murnahan, J.P.; Mow, V.C.; et al. A standardized method for assessment of elbow function. Research Committee, American Shoulder and Elbow Surgeons. J. Shoulder Elb. Surg. 1999, 8, 351–354. [Google Scholar]
- Doornberg, J.N.; Ring, D.; Fabian, L.M.; Malhotra, L.; Zurakowski, D.; Jupiter, J.B. Pain dominates measurements of elbow function and health status. J. Bone Joint Surg. 2005, 87, 1725–1731. [Google Scholar] [CrossRef]
- Gonzales, V.A.; Martelli, M.F.; Baker, J.M. Psychological assessment of persons with chronic pain. NeuroRehabilitation 2000, 4, 69–83. [Google Scholar]
- Knight, J.B.; Callahan, L.F.; Luong, M.L.; Shreffler, J.; Schoster, B.; Renner, J.B.; Jordan, J.M. The association of disability and pain with individual and community socioeconomic status in people with hip osteoarthritis. Open Rheumatol. J. 2011, 5, 51–58. [Google Scholar] [CrossRef]
- Savoie, F.H.; O’Brien, M.J.; Field, L.D. Arthroscopy for Arthritis of the Elbow. Hand Clin. 2011, 27, 171–178. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Morrey, B.F. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J. Bone Joint Surg. 1992, 74, 84–94. [Google Scholar]
- Ward, W.G.; Belhobek, G.H.; Anderson, T.E. Arthroscopic elbow findings: Correlation with preoperative radiographic studies. Arthroscopy 1992, 8, 498–502. [Google Scholar] [CrossRef]
- Savoie, F.H.; Nunley, P.D.; Field, L.D. Arthroscopic management of the arthritic elbow: Indications, technique, and results. J. Shoulder Elb. Surg. 1999, 8, 214–219. [Google Scholar] [CrossRef]
- Van Brakel, R.W.; Eygendaal, D. Intra-articular injection of hyaluronic acid is not effective for the treatment of post-traumatic osteoarthritis of the elbow. Arthroscopy 2006, 22, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A. Management of the patient with rheumatoid arthritis. The role of the hand therapist. Hand Clin. 1989, 5, 291–309. [Google Scholar] [PubMed]
- Wilk, K.E.; Macrina, L.C.; Cain, E.L.; Dugas, J.R.; Andrews, J.R. Rehabilitation of the Overhead Athlete’s Elbow. Sports Health 2012, 4, 404–414. [Google Scholar] [CrossRef]
- Murphy, M.S. Management of inflammatory arthritis around the elbow. Hand Clin. 2002, 18, 161–168. [Google Scholar] [CrossRef]
- Sears, B.W.; Puskas, G.J.; Morrey, M.E.; Sanchez-Sotelo, J.; Morrey, B.F. Posttraumatic Elbow Arthritis in the Young Adult: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2012, 20, 704–714. [Google Scholar] [CrossRef]
- Noticewala, M.S.; Levi, M.A.; Ahmad, C.S.; Levine, W.N.; Jobin, C.M. Arthroscopic Elbow Osteocapsular Arthroplasty. Arthrosc. Tech. 2017, 6, e2111–e2118. [Google Scholar] [CrossRef]
- Sochacki, K.R.; Jack, R.A., 2nd.; Hirase, T.; McCulloch, P.C.; Lintner, D.M.; Liberman, S.R.; Harris, J.D. Arthroscopic Debridement for Primary Degenerative Osteoarthritis of the Elbow Leads to Significant Improvement in Range of Motion and Clinical Outcomes: A Systematic Review. Arthroscopy 2017, 33, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.P.; Redden, J.F.; Stanley, D. Treatment of osteoarthritis of the elbow: A comparison of open and arthroscopic debridement. Arthroscopy 2000, 16, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E.; King, G.J.; Steinmann, S.P.; Cohen, M.S. Elbow arthroscopy: Indications, techniques, outcomes, and complications. Instr. Course Lect. 2015, 64, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.N.; Morrey, B.F. Interposition Arthroplasty with an Achilles Tendon Allograft as a Salvage Procedure for the Elbow. J. Bone Joint Surg. 2008, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, M.; Kwon, Y.W. The rise of the metal elbow. Bull. Hosp. Jt. Dis. 2013, 71, 24–31. [Google Scholar]
- Kwapisz, A.; Prabhakar, S.; Compagnoni, R.; Sibilska, A.; Randelli, P. Platelet-Rich Plasma for Elbow Pathologies: A Descriptive Review of Current Literature. Curr. Rev. Musculoskelet Med. 2018, 11, 598–606. [Google Scholar] [CrossRef] [PubMed]
- De Kesel, R.; Van Glabbeek, F.; Mugenzi, D.; De Vos, J.; Vermeulen, K.; Van Renterghem, D.; Bortier, H.; Schuind, F. Innervation of the elbow joint: Is total denervation possible? A cadaveric anatomic study. Clin. Anat. 2012, 25, 746–754. [Google Scholar] [CrossRef]
- Bachman, D.; Cil, A. Current concepts in elbow arthroplasty. EFORT Open Rev. 2017, 2, 83–88. [Google Scholar] [CrossRef]
- Gardner, O.F.W.; Musumeci, G.; Neumann, A.J.; Eglin, D.; Archer, C.W.; Alini, M.; Stoddart, M.J. Asymmetrical seeding of MSCs into fibrin-poly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis. J. Tissue Eng. Regen. Med. 2017, 11, 2912–2921. [Google Scholar] [CrossRef]
- Valderrabano, V.; Steiger, C. Treatment and Prevention of Osteoarthritis through Exercise and Sports. J. Aging Res. 2010, 2011, 374653. [Google Scholar] [CrossRef]
- Mautner, B.K.; Blazuk, J. Overuse Throwing Injuries in Skeletally Immature Athletes—Diagnosis, Treatment, and Prevention. Curr. Sports Med. Rep. 2015, 14, 209–214. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravalli, S.; Pulici, C.; Binetti, S.; Aglieco, A.; Vecchio, M.; Musumeci, G. An Overview of the Pathogenesis and Treatment of Elbow Osteoarthritis. J. Funct. Morphol. Kinesiol. 2019, 4, 30. https://doi.org/10.3390/jfmk4020030
Ravalli S, Pulici C, Binetti S, Aglieco A, Vecchio M, Musumeci G. An Overview of the Pathogenesis and Treatment of Elbow Osteoarthritis. Journal of Functional Morphology and Kinesiology. 2019; 4(2):30. https://doi.org/10.3390/jfmk4020030
Chicago/Turabian StyleRavalli, Silvia, Carmelo Pulici, Stefano Binetti, Alessandra Aglieco, Michele Vecchio, and Giuseppe Musumeci. 2019. "An Overview of the Pathogenesis and Treatment of Elbow Osteoarthritis" Journal of Functional Morphology and Kinesiology 4, no. 2: 30. https://doi.org/10.3390/jfmk4020030
APA StyleRavalli, S., Pulici, C., Binetti, S., Aglieco, A., Vecchio, M., & Musumeci, G. (2019). An Overview of the Pathogenesis and Treatment of Elbow Osteoarthritis. Journal of Functional Morphology and Kinesiology, 4(2), 30. https://doi.org/10.3390/jfmk4020030