Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC)
Abstract
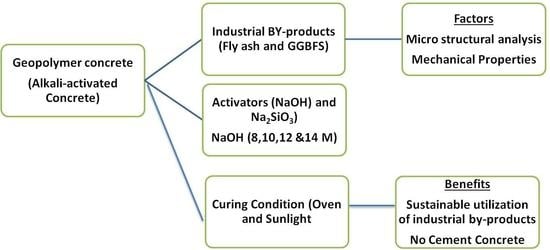
:1. Introduction
Development of Geopolymers
2. Material and Properties
3. Mix Proportions
Standards
4. Geopolymer Binders Synthesized by Fly Ash and Ground Granulated Blast Furnace Slag (GGBFS)
5. Engineering Properties of Geopolymer Concrete
5.1. Compressive Strength
5.2. Split Tensile Strength
5.3. Flexural Strength
6. Conclusions
- The GPC specimens were cast with inorganic materials (fly ash and GGBFS) and cured at a constant temperature of 70 °C for one day then further cured at sunlight for 3, 7, 14 and 28 days. In these curing conditions, the compressive strength of GPC was higher at 70 °C oven curing for 24 h as well as 28 days of sunlight curing, that is 34.15 MPa (for mix M5).
- The maximum split tensile strength was 3.87 MPa obtained at 14 M of NaOH solution and at 70 °C oven curing for 24 h as well as 28 days of sunlight curing. Similarly, the maximum flexural strength value is 11.02 (for mix M5) at the curing of 70 °C in the oven and 28 days of sunlight.
- The increase of molarity of NaOH solution from 8–14 M, the compressive strength, splitting tensile strength and flexural strength of GPC were increased by 33%, 26% and 42.5% respectively.
- Oven curing at higher temperatures was not undertaken according to previous research, and it is better to consider some fiber reinforcement in the geopolymer mix for future studies to improve additional properties; the scope is there for durability analysis at different environmental conditions.
- Geopolymer concrete one of the eco-friendly alternatives to ordinary Portland cement concrete because it utilizes less raw material. For this reason, geopolymer concrete is a strong material that provides sustainable development for infrastructural needs.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Sara banu, J.; Kumutha, R.; Vijai, K. A Review on Durability Studies of Geopolymer Concrete and Mortar under Aggressive Environment. Int. J. Civ. Eng. 2017, 4, 32–35. [Google Scholar]
- Singh, N.B. Fly Ash-Based Geopolymer Binder: A Future Construction Material. Materials 2018, 8, 299. [Google Scholar] [CrossRef]
- Yellaiah, P.; Sharma, S.K.; Rao, T.D.G. Tensile Strength of Fly ash Based Geopolymer Mortar. ARPN J. Eng. Appl. Sci. 2014, 9, 2297–2301. [Google Scholar]
- Roy, D.M.; Jiang, W.; Silsbee, M.R. Chloride Diffusion in Ordinary, Blended, and Alkali-Activated Cement Pastes and its Relation to Other Properties. Cem. Concr. Res. 2000, 30, 1879–1884. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric Materials Prepared Using Class F Fly Ash and Elevated Temperature Curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Farina, I.; Modano, M.; Zuccaro, G.; Goodall, R.; Colangelo, F. Improving flexural strength and toughness of geopolymer mortars through additively manufactured metallic rebars. Compos. Part B 2018, 145, 155–161. [Google Scholar] [CrossRef]
- Davidovits, J. Ancient and Modern Concretes: What is the Real Difference? Concr. Int. 1972, 9, 23–28. [Google Scholar]
- Davidovits, J. Geopolymers and geopolymeric new materials. J. Anal. Calorim. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Davidovits, J. Properties of Geopolymer Cements; Alkali Cements and Concretes: Kiev, Ukraine, 1994. [Google Scholar]
- Davidovits, J. 30 Years of Successes and Failures in Geopolymer Applications. In Proceedings of the Geopolymer 2002 Conference, Melbourne, Australia, 28–29 October 2002. [Google Scholar]
- Duxson, P.; Ferna’ndez-Jime´nez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Duxson, P. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Komljenovi´c, M.; Bascarevic, Z.; Bradic, V. Mechanical and microstructural properties of alkali-activated fly ash geopolymer. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of elevated temperatures geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Sazama, P.; Bortnovsky, O.; Dědeček, J.; Tvarůžková, Z.; Sobalík, Z. Geopolymer based catalysts—New group of catalytic materials. Catal. Today 2011, 164, 92–99. [Google Scholar] [CrossRef]
- Van Deventer, J.S.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement a Review, Published in Geopolymer Science and Technics. Technical Paper #21, Geopolymer Institute Library. 2013. Available online: www.geopolymer.org (accessed on 11 January 2013).
- Zhang, Z. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, B.; Saraswathy, V.; Kwon, S. Strength and Durability Performance of Alkali-Activated Rice Husk Ash Geopolymer Mortar. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Dashti, S. Experimental Evaluation of Beneficial Use of Lake Michigan Dredged Materials in Flyash Based Geopolymer Concrete. Master’s Thesis, University of Wisconsin Milwaukee, Milwaukee, WI, USA, 2015. [Google Scholar]
- Sobolev, K.; Moini, M.; Muzenski, S.; Rani Pradoto, D.; Kozhukhova, M.; Flores-Vivian, I.; Le Pham, S.C.; Faheem, A. Laboratory Study of Optimized Concrete Pavement Mixtures; Wisconsin Highway Research Program: Madison, WI, USA, 2015.
- Naskar, S. Arun Kumar Chakraborty, Effect of nano materials in geopolymer concrete. Perspect. Sci. 2016, 8, 273–275. [Google Scholar] [CrossRef]
- Pan, Z.; Cao, Y.; Wuhrer, R. Measurement and Prediction of Thermal Properties of Alkali-activated Flyash/slag Binders at Elevated Temperatures. Mater. Struct. 2018, 51, 108. [Google Scholar] [CrossRef]
- Aggarwal, P.; Singh, R.P.; Aggarwal, Y. Use of nano-silica in cement based materials—A review. Cogent Eng. 2015, 2, 1078018. [Google Scholar] [CrossRef]
- Gorhan, G.; Kurklu, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Ganapati Naidu, P.; Prasad, A.S.S.N.; Adiseshu, S.; Satayanarayana, P.V.V. A Study on Strength Properties of Geopolymer Concrete with Addition of G.G.B.S. Int. J. Eng. Res. Dev. 2012, 2, 19–28. [Google Scholar]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for Geopolymer pastes in comparison to Ordinary Portland Cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Abdel-Gawwada, H.A.; Abo-El-Eneinb, S.A. A novel method to produce dry geopolymer cement powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Haq, E.U.; Padmanabhan, S.K.; Licciulli, A. Synthesis and characteristics of fly ash and bottom ash based geopolymers—A comparative study. Ceram. Int. 2014, 40, 2965–2971. [Google Scholar] [CrossRef]
- Bakri, A.M.M.A.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Rafiza, A.R.; Zarina, Y. The Processing, Characterization, and Properties of Fly ash Based Geopolymer Concrete. Rev. Adv. Mater. Sci. 2012, 30, 90–97. [Google Scholar]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]







| Cement and Industrial Byproducts | Density (g/cm3) | Specific Surface Area (m2/g) | Particle Size Distribution (mm) |
|---|---|---|---|
| OPC (type I) (I33) | 3.12 | 0.85 | 1.1–50 |
| Class F flyash (G 05) | 2.45 | 0.7 | 2.0–40 |
| Granulated blast-furnace slag (G42) | 2.9 | 0.75 | 0.70–40 |
| Silica fume (G-15) | 2.05 | 18.02 | 0.1–5.0 |
| Author | Year | Significance |
|---|---|---|
| Davidovits [9] | 1972 | Discovery of Geopolymers |
| Davidovits [10] | 1989 | Geopolymeric new materials |
| Davidovits [11] | 1994 | Geopolymer cements |
| Davidovits [12] | 2002 | Successes and Failures in Geopolymer Applications |
| P. Duxson et al. [13] | 2006 | Current state of the art |
| Peter Duxson et al. [14] | 2007 | Geopolymer technology in the development of green concrete |
| Komljenovi M et al. [15] | 2010 | Microstructural properties |
| Kong DLY and Sanjayan JG [16] | 2010 | Structural composites for high temperatures |
| PetrSazama et al. [17] | 2011 | Geopolymer based catalysts |
| Jannie S.J. van Deventer et al. [18] | 2011 | Geopolymer cement |
| Davidovits [19] | 2013 | Foundry Resins (Nano-poly (Silaxilanol)) |
| Zuhua Zhang et al. [20] | 2014 | Geopolymer foam concrete |
| Yun Yong Kim et al. [21] | 2014 | Durability Performance |
| Sara Dashti [22] | 2015 | Use of Lake Michigan Dredged Materials |
| Dr. Konstantin Sobolev et al. [23] | 2015 | Optimized Concrete Pavement |
| Naskar and Chakraborty [24] | 2016 | Nano materials in geopolymer concrete |
| Materials | Specific Gravity |
|---|---|
| Coarse aggregate | 2.71 |
| Fine Aggregate | 2.48 |
| Fly ash | 2.1 |
| Ground Granulated Blast Furnace Slag | 2.22 |
| Density of Sodium Silicates | 1.53 |
| Sodium Hydroxide | 1 |
| Water | 1 |
| Super Plasticizer | 2.12 |
| Materials | Range |
|---|---|
| Alkaline liquids/Binder | 0.3–0.45 |
| Sodium silicate/sodium hydroxide | 2.0–2.5 |
| Water/Binder | 0.16–0.24 |
| Total aggregate in mass of concrete | 65–85% |
| Fine aggregate content in total aggregate | 30% |
| Added water content | 0.02%–0.06% of mass of cementitious material |
| Super Plasticizers | 1.5%–4% of mass of cementitious material |
| Materials used | Quantity |
| Unit weight of concrete | 2400 kg/m3 |
| Percentage of aggregate in total mass of concrete | 70% |
| Aggregate content in total mass of concrete | 1680 kg/m3 |
| Percentage of fine aggregate in total mass of aggregate | 30% |
| Fine aggregate in total mass of aggregate | 504 kg/m3 |
| Coarse aggregate in total mass of aggregate | 1176 kg/m3 |
| Cementitious materials used | |
| Ratio of alkaline liquid to cementitious material | 0.35 |
| Mass of Cementitious material and alkaline liquid | 720 kg/m3 |
| Mass of Cementitious material | 533 kg/m3 |
| Mass of alkaline liquid | 187 kg/m3 |
| Fly ash (30%) | 159.9 kg/m3 |
| GGBFS (70%) | 373.1 kg/m3 |
| Alkaline liquids required | |
| Ratio of sodium silicate and sodium hydroxide | 2.5 |
| Mass of sodium hydroxide | 53 kg/m3 |
| Mass of sodium silicates | 134 kg/m3 |
| Water required in Sodium silicate | |
| Na2O | 15.30% |
| SiO2 | 33.69% |
| H2O | 51.01% |
| Water content in sodium silicate | 69 kg |
| solids content in sodium silicate | 65 kg |
| Water required in sodium hydroxide | |
| Molarity ratio | 6 |
| Mass of NaOH solids | 240 gm |
| NaOH | 24% |
| H2O | 76% |
| Solid content in sodium hydroxide | 13 kg |
| Water content in sodium hydroxide | 40 kg |
| Total water content | 109 kg |
| Total solid content | 611 kg |
| Water to cementitious material ratio | 0.178 |
| Super plasticizer requirement | |
| Super plasticizer | 4% |
| Mass of super plasticizers | 24.44 kg/m3 |
| Percentage of extra water content | 1% |
| Extra water content | 5.33 kg/m3 |
| Material | Absolute Weight of Material | Material | Absolute Weight of Material |
|---|---|---|---|
| Coarse Aggregate (CA) | 433.94 kg | Standard weight | 1000 kg |
| Fine Aggregate (FA) | 203.22 kg | Difference | 38.82 |
| Fly ash | 76.14 kg | Coarse Aggregate Difference | 27.17 |
| GGBFS | 168.06 kg | Fine Aggregate Difference | 11.64 |
| Sodium Silicates | 87.58 kg | Coarse Aggregate corrected | 406.77 |
| Sodium Hydroxide | 53 kg | Fine Aggregate corrected | 191.58 |
| Water | 5.33 kg | Unit weight corrected CA | 1102.36 kg/m3 |
| Super Plasticizers | 11.52 kg | Unit weight corrected FA | 475.12 kg/m3 |
| Absolute Weight | 1038.82 kg | Water to cementitious material ratio | 0.178 |
| Materials (kg) | M1 (6M NaOH, 30% GGBS & 70%FA) | M2 (8M NaOH, 40% GGBS & 60%FA) | M3 (10M NaOH, 50% GGBS & 50%FA) | M4 (12M NaOH, 60% GGBS & 40%FA) | M5 (14M NaOH, 70% GGBS & 30%FA) |
|---|---|---|---|---|---|
| Coarse aggregate | 132.26 | 132.26 | 132.26 | 132.26 | 132.26 |
| Fine aggregate | 57.01 | 57.01 | 57.01 | 57.01 | 57.01 |
| Flyash | 44.87 | 38.46 | 32.05 | 25.64 | 19.23 |
| GGBFS | 19.23 | 25.64 | 32.05 | 38.46 | 44.87 |
| Mass of sodium silicate | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 |
| Total water content in sodium silicate | 8.29 | 8.29 | 8.29 | 8.29 | 8.29 |
| Total sodium silicate | 16.11 | 16.11 | 16.11 | 16.11 | 16.11 |
| Mass of sodium hydroxide | 1.56 | 2.04 | 2.64 | 3.12 | 3.6 |
| Total water content in sodium hydroxide | 4.81 | 4.33 | 3.72 | 3.24 | 2.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellum, R.R.; Nerella, R.; Madduru, S.R.C.; Indukuri, C.S.R. Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC). Infrastructures 2019, 4, 20. https://doi.org/10.3390/infrastructures4020020
Bellum RR, Nerella R, Madduru SRC, Indukuri CSR. Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC). Infrastructures. 2019; 4(2):20. https://doi.org/10.3390/infrastructures4020020
Chicago/Turabian StyleBellum, Ramamohana Reddy, Ruben Nerella, Sri Rama Chand Madduru, and Chandra Sekhar Reddy Indukuri. 2019. "Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC)" Infrastructures 4, no. 2: 20. https://doi.org/10.3390/infrastructures4020020
APA StyleBellum, R. R., Nerella, R., Madduru, S. R. C., & Indukuri, C. S. R. (2019). Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC). Infrastructures, 4(2), 20. https://doi.org/10.3390/infrastructures4020020