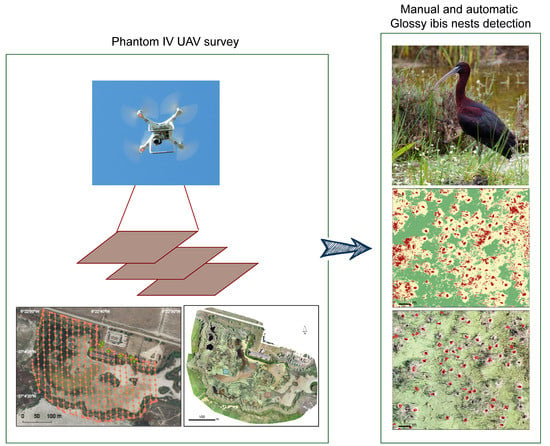
Drone Monitoring of Breeding Waterbird Populations: The Case of the Glossy Ibis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species and Study Area
2.2. Drone Survey Method
2.3. Visual Identification of Breeding Birds
2.4. Automatic Identification of Breeding Birds
2.4.1. Supervised Classification
2.4.2. Image Processing
2.5. Validation
3. Results
3.1. Manual Counting
3.2. Automatic Counting
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Statements
References
- Haase, P.; Tonkin, J.D.; Stoll, S.; Burkhard, B.; Frenzel, M.; Geijzendorffer, I.R.; Hauser, C.; Klotz, S.; Kuhn, I.; McDowell, W.H.; et al. The next generation of site-based long-term ecological monitoring: Linking essential biodiversity variables and ecosystem integrity. Sci. Total Environ. 2018, 613–614, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Burrows, M.T.; Duarte, C.M.; Poloczanska, E.S.; Richardson, A.J.; Schoeman, D.S.; Singer, M.C. Beyond climate change attribution in conservation and ecological research. Ecol. Lett. 2013, 16, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Magurran, A.E.; Baillie, S.R.; Buckland, S.T.; Dick, J.M.; Elston, D.A.; Scott, E.M.; Smith, R.I.; Somerfield, P.J.; Watt, A.D. Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol. Evol. 2010, 25, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Nager, R.G.; Hafner, H.; Johnson, A.R.; Cézilly, F. Environmental Impacts on Wetland Birds: Long-Term Monitoring Programmes in the Camargue, France. Ardea 2010, 98, 309–318. [Google Scholar] [CrossRef]
- Grumbine, R.E. What is ecosystem management? Conserv. Biol. 1994, 8, 27–38. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal species: A multi-species umbrella for nature conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Vos, P.; Meelis, E.; Ter Keurs, W.J. A framework for the design of ecological monitoring programs as a tool for environmental and nature management. Environ. Monit. Assess. 2000, 61, 317–344. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Newton, I.; Green, R. Bird Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2004; Volume 1. [Google Scholar]
- Ramírez, F.; Rodríguez, C.; Seoane, J.; Figuerola, J.; Bustamante, J. How will climate change affect endangered Mediterranean waterbirds? PLOS ONE 2018, 13, e0192702. [Google Scholar] [CrossRef]
- Bako, G.; Tolnai, M.; Takacs, A. Introduction and testing of a monitoring and colony-mapping method for waterbird populations that uses high-speed and ultra-detailed aerial remote sensing. Sensors 2014, 14, 12828–12846. [Google Scholar] [CrossRef]
- Frederick, P.C.; Towles, T.; Sawicki, R.J.; Bancroft, G.T. Comparison of aerial and ground techniques for discovery and census of wading bird (Ciconiiformes) nesting colonies. The Condor 1996, 98, 837–841. [Google Scholar] [CrossRef]
- Kushlan, J.A. Effects of helicopter censuses on wading bird colonies. J. Wildlife Manage. 1979, 43, 756–760. [Google Scholar] [CrossRef]
- Díaz-Delgado, R. An integrated monitoring programme for Doñana Natural Space: The set-up and implementation. In Conservation Monitoring in Freshwater Habitats: A Practical Guide and Case Studies; Hurford, C., Schneider, M., Cowx, I., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 325–337. [Google Scholar]
- Kingsford, R.T.; Porter, J.L. Monitoring waterbird populations with aerial surveys what have we learnt? Wildl. Res. 2009, 36, 29–40. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Sardà-Palomera, F.; Bota, G.; Padilla, N.; Brotons, L.; Sardà, F. Unmanned aircraft systems to unravel spatial and temporal factors affecting dynamics of colony formation and nesting success in birds. J. Avian Biol. 2017, 48, 1273–1280. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Baylis, S.M.; Mott, R.; Herrod, A.; Clarke, R.H. Precision wildlife monitoring using unmanned aerial vehicles. Sci. Rep. 2016, 6, 22574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brisson-Curadeau, E.; Bird, D.; Burke, C.; Fifield, D.A.; Pace, P.; Sherley, R.B.; Elliott, K.H. Seabird species vary in behavioural response to drone census. Sci. Rep. 2017, 7, 17884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-G.; Yoo, S.H.; Kwon, O. Possibility of applying unmanned aerial vehicle (UAV) and mapping software for the monitoring of waterbirds and their habitats. J. Ecol. Environ. 2017, 41. [Google Scholar] [CrossRef] [Green Version]
- Fuller, A.R.; McChesney, G.J.; Golightly, R.T. Aircraft disturbance to Common Murres (Uria aalge) at a breeding colony in Central California, USA. Waterbirds 2018, 41, 257–267. [Google Scholar] [CrossRef]
- Lyons, M.; Brandis, K.; Callaghan, C.; McCann, J.; Mills, C.; Ryall, S.; Kingsford, R. Bird interactions with drones, from individuals to large colonies. Australian Field Ornithol. 2018, 35. [Google Scholar] [CrossRef]
- Descamps, S.; Béchet, A.; Descombes, X.; Arnaud, A.; Zerubia, J. An automatic counter for aerial images of aggregations of large birds. Bird Study 2011, 58, 302–308. [Google Scholar] [CrossRef]
- Grenzdörffer, G.J. UAS-based automatic bird count of a common gull colony. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2013, 1, 169–174. [Google Scholar] [CrossRef]
- Díaz-Delgado, R.; Aragonés, D.; Afán, I.; Bustamante, J. Long-term monitoring of the flooding regime and hydroperiod of Doñana marshes with Landsat time series (1974–2014). Remote Sens. 2016, 8. [Google Scholar] [CrossRef]
- Haberl, H.; Gaube, V.; Díaz-Delgado, R.; Krauze, K.; Neuner, A.; Peterseil, J.; Plutzar, C.; Singh, S.J.; Vadineanu, A. Towards an integrated model of socioeconomic biodiversity drivers, pressures and impacts. A feasibility study based on three European long-term socio-ecological research platforms. Ecol. Econ. 2009, 68, 1797–1812. [Google Scholar] [CrossRef] [Green Version]
- Santoro, S.; Máñez, M.; Green, A.J.; Figuerola, J. Formation and growth of a heronry in a managed wetland in Doñana, southwest Spain. Bird Study 2010, 57, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Santoro, S.; Green, A.J.; Speakman, J.R.; Figuerola, J. Facultative and non-facultative sex ratio adjustments in a dimorphic bird species. Oikos 2015, 124, 1215–1224. [Google Scholar] [CrossRef] [Green Version]
- Ramo, C.; Aguilera, E.; Figuerola, J.; Máñez, M.; Green, A.J. Long-term population trends of colonial wading birds breeding in Doñana (SW Spain) in relation to environmental and anthropogenic factors. Ardeola 2013, 60, 305–326. [Google Scholar] [CrossRef]
- Figuerola, J.; Máñez, M.; Ibáñez, F.; García, L.; Garrido, H. Morito Común, Plegadis falcinellus. In Atlas de las Aves Reproductoras de España; Martí, R., del Moral, J.C., Eds.; Dirección General de Conservación de la Naturaleza-SEO/BirdLife: Madrid, Spain, 2004; pp. 124–125. [Google Scholar]
- Máñez, M.; García, L.; Arroyo, J.L.; Del Valle, J.L.; Rodríguez, R.; Martínez, M.; Chico, A. Twenty-two years of monitoring of the Glossy Ibis (Plegadis falcinellus) in Doñana. In Proceedings of the First International Workshop on Glossy Ibis, Doñana, Spain, 27–29 November 2017. [Google Scholar]
- BirdLife International. Plegadis falcinellus. The IUCN Red List of Threatened Species. 2016. Available online: http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22697422A86436401.en (accessed on 19 November 2018).
- Madroño, A.; González, G.G.; Atienza, J.C. Libro rojo de las aves de España; Dirección General para la Biodiversidad-SEO/BirdLife: Madrid, Spain, 2004. [Google Scholar]
- Del Hoyo, J.; Elliot, A.; Sargatal, J. Handbook of the Birds of the World; Lynx Editions: Barcelona, Spain, 1992. [Google Scholar]
- Valverde, J.A. Vertebrados de las marismas del Guadalquivir (introducción al estudio ecológico); Archivos del Instituto de Aclimatación: Almería, Spain, 1960; Vol. IX. [Google Scholar]
- Cramp, S.; Simmons, K.E.L.; Perrins, C.M. The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1977; Vol. I. [Google Scholar]
- Ivosevic, B.; Han, Y.-G.; Kwon, O. Calculating coniferous tree coverage using unmanned aerial vehicle photogrammetry. J. Ecol. Env. 2017, 41. [Google Scholar] [CrossRef]
- Richards, J.A.; Richards, J.A. Remote Sensing Digital Image Analysis; Springer: Berlin/Heidelberg, Germany, 1999; Volume 3. [Google Scholar]
- Pal, M. Random forest classifier for remote sensing classification. Int. J. Remote Sens. 2007, 26, 217–222. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 22 October 2018).
- Bivand, R.; Keitt, T.K.; Rowlingson, B. rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library, R package version 1.2-16; 2018. Available online: https://cran.r-project.org/web/packages/rgdal/index.html (accessed on 22 October 2018).
- Hijmans, R.J. raster: Geographic Data Analysis and Modeling, R package version 2.6-7; 2017. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 22 October 2018).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C. caret: Classification and Regression Training, R package version 6.0-80; 2012. Available online: https://cran.r-project.org/web/packages/caret/index.html (accessed on 22 October 2018).
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: an open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulava, S.; Bean, W.T.; Richmond, O.M. Environmental reviews and case studies: Applications of unmanned aircraft systems (UAS) for waterbird surveys. Environ. Pract. 2015, 17, 201–210. [Google Scholar] [CrossRef]
- Barr, J.R.; Green, M.C.; DeMaso, S.J.; Hardy, T.B. Detectability and visibility biases associated with using a consumer-grade unmanned aircraft to survey nesting colonial waterbirds. J. Field Ornithol. 2018, 89, 242–257. [Google Scholar] [CrossRef]
- Mallard, F.; Le Bourlot, V.; Tully, T. An automated image analysis system to measure and count organisms in laboratory microcosms. PLOS ONE 2013, 8, e64387. [Google Scholar] [CrossRef] [PubMed]
- Trathan, P.N. Image analysis of color aerial photography to estimate penguin population size. Wildlife Soc. B. 2004, 32, 332–343. [Google Scholar] [CrossRef]
- Hurford, C. Improving the accuracy of bird counts using manual and automated counts in ImageJ: An open-source image processing program. In The Roles of Remote Sensing in Nature Conservation: A Practical Guide And Case Studies; Díaz-Delgado, R., Lucas, R., Hurford, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 249–276. [Google Scholar] [CrossRef]
- Chabot, D.; Francis, C.M. Computer-automated bird detection and counts in high-resolution aerial images: A review. J. Field Ornithol. 2016, 87, 343–359. [Google Scholar] [CrossRef]
- Liu, C.-C.; Chen, Y.-H.; Wen, H.-L. Supporting the annual international black-faced spoonbill census with a low-cost unmanned aerial vehicle. Ecol. Inform. 2015, 30, 170–178. [Google Scholar] [CrossRef]
- Díaz-Delgado, R.; Máñez, M.; Martínez, A.; Canal, D.; Ferrer, M.; Aragonés, D. Using UAVs to map aquatic bird colonies. In The Roles of Remote Sensing in Nature Conservation; Springer: Cham, Switzerland, 2017; pp. 277–291. [Google Scholar]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal. Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]





| Area | Nest Density (Nests m−2) | Nest Distance (m) | Nest Distance (Range, m) | Number of Nests |
|---|---|---|---|---|
| Total colony | 0.22 | 0.88 ± 0.48 | 0.26–7.84 | 7134 |
| Typha spp. | 0.41 ± 0.22 | 0.82 ± 0.36 | 0.32–3.85 | 94–228 |
| Lemna spp. | 0.43 ± 0.05 | 0.79 ± 0.42 | 0.33–3.35 | 128–161 |
| Area | Accuracy (%) | Commission Error (%) | Omission Error (%) | Number of Nests |
|---|---|---|---|---|
| Total colony | 46.37 | 66.61 | 53.63 | 7134 |
| Typha spp. | 50.37 ± 2.19 | 55.06 ± 27.79 | 49.63 ± 2.19 | 94–228 |
| Lemna spp. | 49.01 ± 19.22 | 22.64 ± 7.45 | 50.99 ± 19.22 | 128–161 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afán, I.; Máñez, M.; Díaz-Delgado, R. Drone Monitoring of Breeding Waterbird Populations: The Case of the Glossy Ibis. Drones 2018, 2, 42. https://doi.org/10.3390/drones2040042
Afán I, Máñez M, Díaz-Delgado R. Drone Monitoring of Breeding Waterbird Populations: The Case of the Glossy Ibis. Drones. 2018; 2(4):42. https://doi.org/10.3390/drones2040042
Chicago/Turabian StyleAfán, Isabel, Manuel Máñez, and Ricardo Díaz-Delgado. 2018. "Drone Monitoring of Breeding Waterbird Populations: The Case of the Glossy Ibis" Drones 2, no. 4: 42. https://doi.org/10.3390/drones2040042
APA StyleAfán, I., Máñez, M., & Díaz-Delgado, R. (2018). Drone Monitoring of Breeding Waterbird Populations: The Case of the Glossy Ibis. Drones, 2(4), 42. https://doi.org/10.3390/drones2040042