Composite Films of Waterborne Polyurethane and Few-Layer Graphene—Enhancing Barrier, Mechanical, and Electrical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the Few-Layer/Waterborne Polyurethane (WPU) Composite Films
3. Results
3.1. Reinforcing Materials—Graphite and Few-Layer Graphene
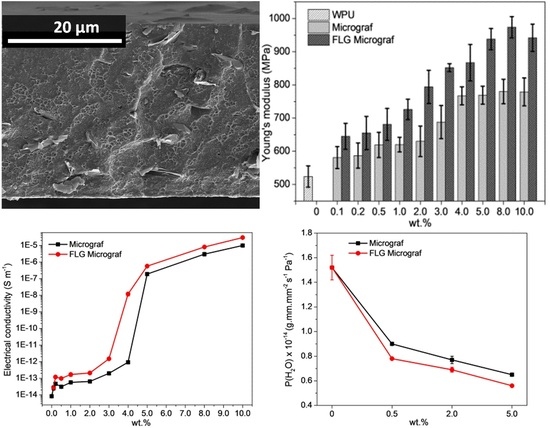
3.2. Nanocomposite Films—Low Composition Range
3.3. Nanocomposite Films—High Composition Range
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chattopadhyay, D.K.; Raju, K.V. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Crawford, D.M.; Escarsega, J.A. Dynamic mechanical analysis of novel polyurethane coating for military applications. Thermochim. Acta 2000, 357, 161–168. [Google Scholar] [CrossRef]
- Hu, J.L.; Meng, H.P.; Li, G.Q.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Lomax, G.R. Breathable polyurethane membranes for textile and related industries. J. Mater. Chem. 2007, 17, 2775–2784. [Google Scholar] [CrossRef]
- Nasar, A.S.; Srinivasan, G.; Mohan, R.; Radhakrishnan, G. Polyurethane solvent-based adhesives for footwear applications. J. Adhes. 1998, 68, 21–29. [Google Scholar] [CrossRef]
- Xiang, C.; Cox, P.J.; Kukovecz, A.; Genorio, B.; Hashim, D.P.; Yan, Z.; Peng, Z.; Hwang, C.C.; Ruan, G.; Samuel, E.L.; et al. Functionalized low defect graphene nanoribbons and polyurethane composite film for improved gas barrier and mechanical performances. ACS Nano 2013, 7, 10380–10386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.M.; Li, M.; Peng, H.X. Functionalized mwnt-doped thermoplastic polyurethane nanocomposites for aerospace coating applications. Macromol. Mater. Eng. 2010, 295, 838–845. [Google Scholar] [CrossRef]
- Noble, K.L. Waterborne polyurethanes. Prog. Org. Coat. 1997, 32, 131–136. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, X.W.; Zhang, Y.Y.; Liu, Z.M.; Jiang, L.; Lei, J.X. Preparation of waterborne polyurethanes based on the organic solvent-free process. Green Chem. 2016, 18, 412–416. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.Q.; Li, S.J.; Cheng, Y.L.; Lei, W.Q.; Meng, X.J. Recent advances in synthesis of waterborne polyurethane and their application in water-based ink: A review. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Krol, P.; Krol, B.; Zenker, M.; Subocz, J. Composites prepared from the waterborne polyurethane cationomers-modified graphene. Part ii. Electrical properties of the polyurethane films. Colloid Polym. Sci. 2015, 293, 2941–2947. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, D.H.; Raghu, A.V.; Reddy, K.R.; Lee, H.I.; Yoon, K.S.; Jeong, H.M.; Kim, B.K. Properties of graphene/waterborne polyurethane nanocomposites cast from colloidal dispersion mixtures. J. Macromol. Sci. B 2012, 51, 197–207. [Google Scholar] [CrossRef]
- Lee, Y.R.; Raghu, A.V.; Jeong, H.M.; Kim, B.K. Properties of waterborne polyurethane/functionalized graphene sheet nanocomposites prepared by an in situ method. Macromol. Chem. Phys. 2009, 210, 1247–1254. [Google Scholar] [CrossRef]
- Raghu, A.V.; Lee, Y.R.; Jeong, H.M.; Shin, C.M. Preparation and physical properties of waterborne polyurethane/functionalized graphene sheet nanocomposites. Macromol. Chem. Phys. 2008, 209, 2487–2493. [Google Scholar] [CrossRef]
- Ding, J.N.; Fan, Y.; Zhao, C.X.; Liu, Y.B.; Yu, C.T.; Yuan, N.Y. Electrical conductivity of waterborne polyurethane/graphene composites prepared by solution mixing. J. Compos. Mater. 2012, 46, 747–752. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, B.K. Synthesis and properties of silanized waterborne polyurethane/graphene nanocomposites. Colloid Polym. Sci. 2014, 292, 51–58. [Google Scholar] [CrossRef]
- Li, P.L.; Ren, H.; Qiu, F.X.; Xu, J.C.; Yu, Z.P.; Yang, P.F.; Xu, B.B.; Jiang, Y.; Yang, D.Y. Preparation and properties of graphene oxide-modified waterborne polyurethane-acrylate hybrids. Polym. Plast. Technol. 2014, 53, 1408–1416. [Google Scholar] [CrossRef]
- Wu, S.L.; Shi, T.J.; Zhang, L.Y. Latex co-coagulation approach to fabrication of polyurethane/graphene nanocomposites with improved electrical conductivity, thermal conductivity, and barrier property. J. Appl. Polym. Sci. 2016, 133, 13. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.B.; Aboutalebi, S.H.; Sharif, F.; Kim, J.K. Self-alignment and high electrical conductivity of ultralarge graphene oxide-polyurethane nanocomposites. J. Mater. Chem. 2012, 22, 12709–12717. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.B.; Lin, X.Y.; Shen, X.; Jia, J.J.; Sharif, F.; Kim, J.K. Highly aligned, ultralarge-size reduced graphene oxide/polyurethane nanocomposites: Mechanical properties and moisture permeability. Compos. Part A 2013, 49, 42–50. [Google Scholar] [CrossRef]
- Luo, X.M.; Zhang, P.; Ren, J.; Liu, R.; Feng, J.Y.; Ge, B.H. Preparation and properties of functionalized graphene/waterborne polyurethane composites with highly hydrophobic. J. Appl. Polym. Sci. 2015, 132, 8. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.Z.; Qiu, H.X.; Dai, Y.G.; Zheng, Q.B.; Li, J.; Yang, J.H. Self-aligned graphene as anticorrosive barrier in waterborne polyurethane composite coatings. J. Mater. Chem. A 2014, 2, 14139–14145. [Google Scholar] [CrossRef]
- Lei, L.; Xia, Z.B.; Zhang, L.; Zhang, Y.H.; Zhong, L. Preparation and properties of amino-functional reduced graphene oxide/waterborne polyurethane hybrid emulsions. Prog. Org. Coat. 2016, 97, 19–27. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.Y.; Song, L.; Yang, H.Y.; Hu, Y.; Yeoh, G.H. Fabrication and characterization of graphene-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method. Surf. Coat. Technol. 2012, 206, 4778–4784. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.D.; Zhang, Y.D.; Yu, L.G.; Zhang, Z.J. Graphene oxides reduced and modified by hydramines—Potentials as electrode materials of supercapacitors and reinforcing agents of waterborne polyurethane. Mater. Res. Bull. 2014, 59, 117–124. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Ma, C.C.; Tien, H.W.; Liao, W.H.; Wang, Y.S.; Li, S.M.; Yang, C.Y.; Lin, S.C.; Yang, R.B. Effect of covalent modification of graphene nanosheets on the electrical property and electromagnetic interference shielding performance of a water-borne polyurethane composite. ACS Appl. Mater. Interfaces 2015, 7, 2817–2826. [Google Scholar] [CrossRef]
- Hsiao, S.T.; Ma, C.C.M.; Tien, H.W.; Liao, W.H.; Wang, Y.S.; Li, S.M.; Huang, Y.C. Using a non-covalent modification to prepare a high electromagnetic interference shielding performance graphene nanosheet/water-borne polyurethane composite. Carbon 2013, 60, 57–66. [Google Scholar] [CrossRef]
- Lin, S.C.; Ma, C.C.M.; Hsiao, S.T.; Wang, Y.S.; Yang, C.Y.; Liao, W.H.; Li, S.M.; Wang, J.A.; Cheng, T.Y.; Lin, C.W.; et al. Electromagnetic interference shielding performance of waterborne polyurethane composites filled with silver nanoparticles deposited on functionalized graphene. Appl. Surf. Sci. 2016, 385, 436–444. [Google Scholar] [CrossRef]
- Luo, X.M.; Zhang, P.; Liu, R.; Li, W.H.; Ge, B.H.; Cao, M. Preparation and physical properties of functionalized graphene/waterborne polyurethane uv-curing composites by click chemistry. Polym. Int. 2016, 65, 415–422. [Google Scholar] [CrossRef]
- Tian, K.H.; Su, Z.; Wang, H.; Tian, X.Y.; Huang, W.Q.; Xiao, C. N-doped reduced graphene oxide/waterborne polyurethane composites prepared by in situ chemical reduction of graphene oxide. Compos. Part A 2017, 94, 41–49. [Google Scholar] [CrossRef]
- Hu, X.L.; Tian, M.W.; Qu, L.J.; Zhu, S.F.; Han, G.T. Multifunctional cotton fabrics with graphene/polyurethane coatings with far-infrared emission, electrical conductivity, and ultraviolet-blocking properties. Carbon 2015, 95, 625–633. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Evaluation of laundering durability of electro-conductive textile dip-coated on para aramid knit with graphene/waterborne polyurethane composite. Fiber Polym. 2018, 19, 2351–2358. [Google Scholar] [CrossRef]
- Cai, K.W.; Zuo, S.X.; Luo, S.P.; Yao, C.; Liu, W.J.; Ma, J.F.; Mao, H.H.; Li, Z.Y. Preparation of polyaniline/graphene composites with excellent anti-corrosion properties and their application in waterborne polyurethane anticorrosive coatings. RSC Adv. 2016, 6, 95965–95972. [Google Scholar] [CrossRef]
- Christopher, G.; Kulandainathan, M.A.; Harichandran, G. Comparative study of effect of corrosion on mild steel with waterborne polyurethane dispersion containing graphene oxide versus carbon black nanocomposites. Prog. Org. Coat. 2015, 89, 199–211. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.C.; Yang, J.Y.; Li, Y.Y.; Qiu, H.X.; Yang, J.H. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.H.; Wen, S.G.; Yu, D.Y.; Wu, Y.T.; Sun, K. Improved corrosion resistance based on aptes-grafted reduced sulfonated graphene/waterborne polyurethane coatings. J. Coat. Technol. Res. 2018, 15, 1107–1115. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, F. Self-assembled fabrication and flame-retardant properties of reduced graphene oxide/waterborne polyurethane nanocomposites. J. Therm. Anal. Calorim 2014, 118, 1561–1568. [Google Scholar] [CrossRef]
- Cunha, E.; Proenca, M.F.; Pereira, M.G.; Fernandes, M.J.; Young, R.J.; Strutynski, K.; Melle-Franco, M.; Gonzalez-Debs, M.; Lopes, P.E.; Paiva, M.D.C. Water dispersible few-layer graphene stabilized by a novel pyrene derivative at micromolar concentration. Nanomaterials 2018, 8, 675. [Google Scholar]
- Viinikanoja, A.; Kauppila, J.; Damlin, P.; Makila, E.; Leiro, J.; Aaritalo, T.; Lukkari, J. Interactions between graphene sheets and ionic molecules used for the shear-assisted exfoliation of natural graphite. Carbon 2014, 68, 195–209. [Google Scholar] [CrossRef]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.W.; Li, C.; Shi, G.Q. An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Ma, H.L.; Zhang, Q.L.; Peng, J.; Li, J.Q.; Zhai, M.L.; Yu, Z.Z. Facile synthesis of well-dispersed graphene by gamma-ray induced reduction of graphene oxide. J. Mater. Chem. 2012, 22, 13064–13069. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Y.; Zhao, Y.; Chen, D.; Ye, J.; Yao, Y.; Gao, F.; Ni, Z.; Yu, T.; Shen, Z.; et al. Symmetry breaking of graphene monolayers by molecular decoration. Phys. Rev. Lett. 2009, 102, 135501. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Wang, R.G.; Yang, F.; Hao, L.F.; Jiao, W.C.; Liu, W.B. The role of functional groups on graphene oxide in epoxy nanocomposites. Polymer 2013, 54, 5821–5829. [Google Scholar] [CrossRef]
- Damari, S.P.; Cullari, L.; Nadiv, R.; Nir, Y.; Laredo, D.; Grunlan, J.; Regev, O. Graphene-induced enhancement of water vapor barrier in polymer nanocomposites. Compos. Part B-Eng. 2018, 134, 218–224. [Google Scholar] [CrossRef]
- Fredrickson, G.H.; Bicerano, J. Barrier properties of oriented disk composites. J. Chem. Phys. 1999, 110, 2181–2188. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.Y.; Feng, X.M.; Yu, B.; Song, L.; Yeoh, G.H.; Hu, Y. Enhanced mechanical and barrier properties of polyurethane nanocomposite films with randomly distributed molybdenum disulfide nanosheets. Compos. Sci. Technol. 2016, 127, 142–148. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, E.; Paiva, M.C. Composite Films of Waterborne Polyurethane and Few-Layer Graphene—Enhancing Barrier, Mechanical, and Electrical Properties. J. Compos. Sci. 2019, 3, 35. https://doi.org/10.3390/jcs3020035
Cunha E, Paiva MC. Composite Films of Waterborne Polyurethane and Few-Layer Graphene—Enhancing Barrier, Mechanical, and Electrical Properties. Journal of Composites Science. 2019; 3(2):35. https://doi.org/10.3390/jcs3020035
Chicago/Turabian StyleCunha, Eunice, and Maria C. Paiva. 2019. "Composite Films of Waterborne Polyurethane and Few-Layer Graphene—Enhancing Barrier, Mechanical, and Electrical Properties" Journal of Composites Science 3, no. 2: 35. https://doi.org/10.3390/jcs3020035
APA StyleCunha, E., & Paiva, M. C. (2019). Composite Films of Waterborne Polyurethane and Few-Layer Graphene—Enhancing Barrier, Mechanical, and Electrical Properties. Journal of Composites Science, 3(2), 35. https://doi.org/10.3390/jcs3020035