Preparation of Piezo-Resistive Materials by Combination of PP, SEBS and Graphene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
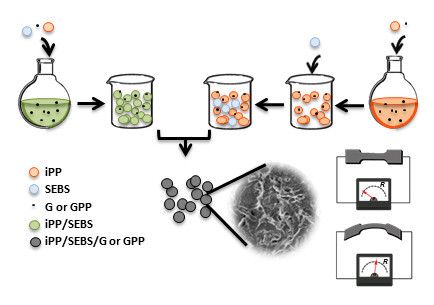
2.2. Preparation of the Nanocomposites
2.3. Characterization
3. Results and Discussion
3.1. Morphology
3.2. Thermal Properties
3.3. Mechanical Properties
3.4. Resistivity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feldman, D. Polyolefin, olefin copolymers and polyolefin polyblend nanocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2016, 53, 651–658. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Srinivasa Rao, G.S.; Mathur, A.B.; Jasra, R. Polyolefin/graphene nanocomposites: A review. RSC Adv. 2017, 7, 23615–23632. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene–polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Marsden, A.J.; Papageorgiou, D.G.; Vallés, C.; Liscio, A.; Palermo, V.; Bissett, M.A.A.; Young, R.J.; Kinloch, I.A. Electrical percolation in graphene-polymer composites. 2D Mater. 2018, 5, 032003. [Google Scholar] [CrossRef]
- Castelaín, M.; Martínez, G.; Marco, C.; Ellis, G.; Salavagione, H.J. Effect of Click-Chemistry Approaches for Graphene Modification on the Electrical, Thermal, and Mechanical Properties of Polyethylene/Graphene Nanocomposites. Macromolecules 2013, 46, 8980–8987. [Google Scholar] [CrossRef] [Green Version]
- Flores, A.; Ania, F.; Salavagione, H.J.; Ellis, G.; Saurel, D.; Gómez-Fatou, M.A. Local mechanical properties of graphene/polyethylene-based nanocomposites by depth-sensing indentation. Eur. Polym. J. 2016, 74, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Quiles-Díaz, S.; Enrique-Jimenez, P.; Papageorgiou, D.G.; Ania, F.; Flores, A.; Kinloch, I.A.; Gómez-Fatou, M.A.; Young, R.J.; Salavagione, H.J. Influence of the chemical functionalization of graphene on the properties of polypropylene-based nanocomposites. Compos. Part Appl. Sci. Manuf. 2017, 100, 31–39. [Google Scholar] [CrossRef]
- Enrique-Jimenez, P.; Quiles-Díaz, S.; Salavagione, H.J.; Fernández-Blázquez, J.P.; Monclús, M.A.; Guzman de Villoria, R.; Gómez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation mapping of multiscale composites of graphene-reinforced polypropylene and carbon fibres. Compos. Sci. Technol. 2019, 169, 151–157. [Google Scholar] [CrossRef]
- Lou, C.-W.; Huang, C.-L.; Pan, Y.-J.; Lin, Z.-I.; Song, X.-M.; Lin, J.-H. Crystallization, mechanical, and electromagnetic properties of conductive polypropylene/SEBS composites. J. Polym. Res. 2016, 23, 84. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Joseph, G.; Shinu, K.P.; Sreejesh, P.R.; Jose, S.; Salim, N.V.; Hameed, N. The role of SEBS in tailoring the interface between the polymer matrix and exfoliated graphene nanoplatelets in hybrid composites. Mater. Chem. Phys. 2015, 163, 182–189. [Google Scholar] [CrossRef]
- Song, P.; Cao, Z.; Cai, Y.; Zhao, L.; Fang, Z.; Fu, S. Fabrication of exfoliated graphene based polypropylene nanocomposites with enhanced mechanical and thermalproperties. Polymer 2011, 52, 4001–4010. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Joseph, G.; Shinu, K.P.; Jose, S.; Salim, N.V.; Hameed, N. Development of hybrid composites for automotive applications: Effect of addition of SEBS on the morphology, mechanical, viscoelastic, crystallization and thermal degradation properties of PP/PS–xGnP composites. RSC Adv. 2015, 5, 255634. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Galeski, A. Strength and toughness of crystalline polymer systems. Prog. Polym. Sci. 2003, 28, 1643–1699. [Google Scholar] [CrossRef]
- Liang, J.Z.; Li, R.K.Y. Rubber toughening in polypropylene: A review. J. Appl. Polym. Sci. 2000, 77, 409–417. [Google Scholar] [CrossRef]
- Setz, S.; Stricker, F.; Kressler, J.; Duschek, T.; Mülhaupt, R. Morphology and mechanical properties of blends of isotactic or syndiotactic polypropylene with SEBS block copolymers. J. Appl. Polym. Sci. 1996, 59, 1117–1128. [Google Scholar] [CrossRef]
- Fanegas, N.; Gomez, M.A.; Jimenez, I.; Marco, C.; Garcia-Martinez, J.M.; Ellis, G. Optimizing the balance between impact strength and stiffness in polypropylene/elastomer blends by incorporation of a nucleating agent. Polym. Eng. Sci. 2008, 48, 80–87. [Google Scholar] [CrossRef]
- Martín, Z.; Jiménez, I.; Gómez, M.Á.; Ade, H.; Kilcoyne, D.A. Interfacial Interactions in PP/MMT/SEBS Nanocomposites. Macromolecules 2010, 43, 448–453. [Google Scholar] [CrossRef]
- Ma, C.G.; Mai, Y.I.; Rong, M.Z.; Ruan, W.H.; Zhang, M.Q. Phase structure and mechanical properties of ternary polypropylene/elastomer/nano-CaCO3 composites. Compos. Sci. Technol. 2007, 67, 2997–3005. [Google Scholar] [CrossRef]
- Fan, B.; Wiwattananukul, R.; Yamaguchi, M. Effect of mixing temperature on the carbon nanofiller distribution in immiscible blends of polycarbonate and polyolefin. Eur. Polym. J. 2017, 96, 295–303. [Google Scholar] [CrossRef]
- Salehiyan, R.; Ray, S.S. Tuning the Conductivity of Nanocomposites through Nanoparticle Migration and Interface Crossing in Immiscible Polymer Blends: A Review on Fundamental Understanding. Macromol. Mater. Eng. 2019, 304, 1800431. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef] [Green Version]
- Castelaín, M.; Salavagione, H.J.; Gómez, R.; Segura, J.L. Supramolecular assembly of graphene with functionalized poly(fluorene-alt-phenylene): The role of the anthraquinone pendant groups. Chem. Commun. 2011, 47, 7677–7679. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Quiles-Díaz, S.; Enrique-Jimenez, P.; Martínez, G.; Ania, F.; Flores, A.; Gómez-Fatou, M.A. Development of Advanced Elastomeric Conductive Nanocomposites by Selective Chemical Affinity of Modified Graphene. Macromolecules 2016, 49, 4948–4956. [Google Scholar] [CrossRef] [Green Version]
- Enrique-Jimenez, P.; Quiles-Díaz, S.; Salavagione, H.J.; Wesner, D.; Schönherr, H.; González-Casablanca, J.; García-Quismondo, R.; Martínez, G.; Gómez-Fatou, M.A.; Ania, F.; et al. Control of the structure and properties of SEBS nanocomposites via chemical modification of graphene with polymer brushes. Eur. Polym. J. 2017, 97, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Quiles-Díaz, S.; Martínez, G.; Gómez-Fatou, M.A.; Ellis, G.J.; Salavagione, H.J. Anhydride-based chemistry on graphene for advanced polymeric materials. RSC Adv. 2016, 6, 36656–36660. [Google Scholar] [CrossRef] [Green Version]





| Method | Sample | Ti (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|---|
| R1 | iPP/SEBS | 384.5 | 453.6 | 0.5 |
| iPP/SEBS/G | 401.9 | 468.7 | 4.8 * | |
| iPP/SEBS/GPP | 400.1 | 467.4 | 5.0 * | |
| R2 | iPP/SEBS | 376.1 | 450.0 | 0.6 |
| iPP/SEBS/G | 400.5 | 464.9 | 4.9 * | |
| iPP/SEBS/GPP | 400.7 | 469.7 | 4.8 * |
| Method | Sample | E (MPa) | εb (%) | σ (MPa) | T (MJ·m−3) | SEBS Mean Area (μm2) |
|---|---|---|---|---|---|---|
| iPP * | 660 ± 30 | 11.5 ± 1.0 | 24.0 ± 7.0 | 1.3 ± 0.2 | ||
| R1 | iPP/SEBS | 652 ± 31 | 450.1 ± 28.0 | 21.7 ± 1.7 | 97.3 ± 6,4 | 1.20 ± 0.20 |
| iPP/SEBS/G | 693 ± 7 | 5.3 ± 1.3 | 23.1 ± 1.5 | 0.9 ± 0.1 | 0.20 ± 0.03 | |
| iPP/SEBS/GPP | 701 ± 22 | 4.2 ± 0.5 | 20.9 ± 1.0 | 0.6 ± 0.1 | 0.25 ± 0.04 | |
| R2 | iPP/SEBS | 622 ± 27 | 55.0 ± 8.0 | 21.0 ± 0.4 | 11.1 ± 1.1 | 1.50 ± 0.30 |
| iPP/SEBS/G | 690 ± 9 | 17.2 ± 2.5 | 26.6 ± 0.6 | 4.0 ± 0.5 | 0.20 ± 0.03 | |
| iPP/SEBS/GPP | 704 ± 22 | 10.0 ± 2.3 | 23.6 ± 0.8 | 1.9 ± 0.4 | 0.31 ± 0.05 |
| Sample | SoM | SoM/SSM |
|---|---|---|
| iPP/SEBS/G | 5.2 × 10−3 | 6.8 × 10−3 |
| iPP/SEBS/GPP | 1.9 × 10−3 | 1.9 × 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyler, H.; Gómez-Fatou, M.A.; Salavagione, H.J. Preparation of Piezo-Resistive Materials by Combination of PP, SEBS and Graphene. J. Compos. Sci. 2019, 3, 37. https://doi.org/10.3390/jcs3020037
Seyler H, Gómez-Fatou MA, Salavagione HJ. Preparation of Piezo-Resistive Materials by Combination of PP, SEBS and Graphene. Journal of Composites Science. 2019; 3(2):37. https://doi.org/10.3390/jcs3020037
Chicago/Turabian StyleSeyler, Helga, Marián A. Gómez-Fatou, and Horacio J. Salavagione. 2019. "Preparation of Piezo-Resistive Materials by Combination of PP, SEBS and Graphene" Journal of Composites Science 3, no. 2: 37. https://doi.org/10.3390/jcs3020037
APA StyleSeyler, H., Gómez-Fatou, M. A., & Salavagione, H. J. (2019). Preparation of Piezo-Resistive Materials by Combination of PP, SEBS and Graphene. Journal of Composites Science, 3(2), 37. https://doi.org/10.3390/jcs3020037