Animal Biopolymer-Plant Biomass Composites: Synergism and Improved Sorption Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
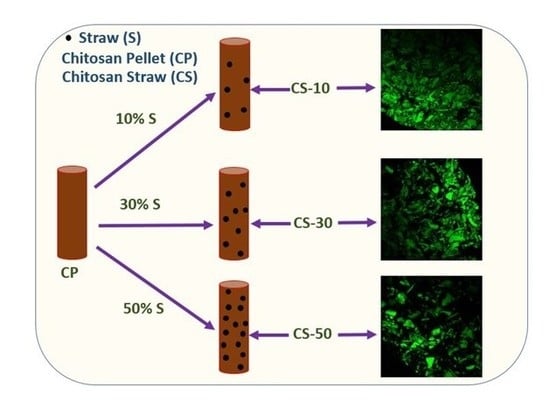
2.2.1. Pellet Preparation
2.2.2. Pellet Characterization
FTIR Spectroscopy
Thermal Gravimetric Analysis (TGA)
Differential Scanning Calorimetry (DSC) Studies
13C Solid State NMR Spectroscopy
Confocal Laser Scanning Microscopy (CLSM)
2.2.3. Sorption studies
Equilibrium Sorption Studies
Kinetic Sorption Studies
3. Results and Discussion
3.1. FT-IR
3.2. 13C Solid State NMR Spectroscopy
3.3. Thermal Analysis
3.4. Confocal Laser Scanning Microscopy (CLSM)
3.5. Sorption Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Parmar, K. Biomass—An Overview on Composition Characteristics and Properties. IRA-Int. J. Appl. Sci. 2017, 7, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105–5116. [Google Scholar] [CrossRef] [Green Version]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Sangon, S.; Hunt, A.; Attard, T.; Mengchang, P.; Ngernyen, Y.; Supanchaiyamat, N. Valorisation of waste rice straw for the production of highly effective carbon based adsorbents for dyes removal. J. Clean. Prod. 2017, 172, 1128–1139. [Google Scholar] [CrossRef]
- Romar-Gasalla, A.; Coelho, F.G.; Nóvoa-Muñoz, C.J.; Arias-Estévez, M.; Fernández-Sanjurjo, J.M.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Wheat Straw as a Bio-Sorbent for Arsenate, Chromate, Fluoride, and Nickel. Water 2017, 9, 690. [Google Scholar] [CrossRef] [Green Version]
- Kyzas, Z.G.; Bikiaris, N.D. Recent Modifications of Chitosan for Adsorption Applications: A Critical and Systematic Review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Pirbazari, A.E. Surfactant-Modified Wheat Straw: Preparation, Characterization and its Application for Methylene Blue Adsorption from Aqueous Solution. J. Chem. Eng. Process Technol. 2015, 6, 231. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Phosphate uptake studies of cross-linked chitosan bead materials. J. Colloid Interface Sci. 2017, 485, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Park, S.-S.; Cho, S.-Y. Adsorption characteristics of Reactive Black 5 onto chitosan beads cross-linked with epichlorohydrin. J. Ind. Eng. Chem. 2012, 18, 1458–1464. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Thermal Properties of Multilayer Nanocomposites Based on Halloysite Nanotubes and Biopolymers. J. Compos. Sci. 2018, 2, 41. [Google Scholar] [CrossRef] [Green Version]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite nanotubes sandwiched between chitosan layers: Novel bionanocomposites with multilayer structures. New J. Chem. 2018, 42, 8384–8390. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/shell gel beads with embedded halloysite nanotubes for controlled drug release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Xie, S.; Cao, J.; Ge, H.; Xu, M.; Zhang, L.; Zhou, J. Quaternized Chitosan/Poly(acrylic acid) Polyelectrolyte Complex Hydrogels with Tough, Self-Recovery, and Tunable Mechanical Properties. Macromolecules 2016, 49, 1049–1059. [Google Scholar] [CrossRef]
- Shankar Tumuluru, J.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. REVIEW: A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.R.; Marques, J.S.; Fonseca, J.L.C. Biocomposites based on chitosan and carnauba straw powder. Polímeros 2014, 24, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Wang, G.; Wang, D.; Wang, Z.; He, C.; Wu, H.; Liu, W. Co-pelletization of torrefied wheat straw and peanut shells. Bioresour. Technol. 2017, 233, 373–381. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J. V Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan–Cellulose Composites and Their Anion Uptake Properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D.; Headley, J. V Fractionation of carboxylate anions from aqueous solution using chitosan cross-linked sorbent materials. RSC Adv. 2015, 5, 82065–82077. [Google Scholar] [CrossRef]
- Zheng, A.; Jiang, L.; Zhao, Z.; Huang, Z.; Zhao, K.; Wei, G.; Wang, X.; He, F.; Li, H. Impact of Torrefaction on the Chemical Structure and Catalytic Fast Pyrolysis Behavior of Hemicellulose, Lignin, and Cellulose. Energy Fuels 2015, 29, 8027–8034. [Google Scholar] [CrossRef]
- Pašalić, H.; Tunega, D.; Aquino, A.J.A.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. The stability of the acetic acid dimer in microhydrated environments and in aqueous solution. Phys. Chem. Chem. Phys. 2012, 14, 4162–4170. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.S. 9-Techniques for analyzing biomaterial surface structure, morphology and topography. In Woodhead Publishing Series in Biomaterials; Williams, R., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 232–255. ISBN 978-1-84569-640-5. [Google Scholar]
- Hurst, G.A.; Novakovic, K. A facile in situ morphological characterization of smart genipin-crosslinked chitosan–poly(vinyl pyrrolidone) hydrogels. J. Mater. Res. 2013, 28, 2401–2408. [Google Scholar] [CrossRef]
- Zu, Y.; Bi, J.; Yan, H.; Wang, H.; Song, Y.; Zhu, B.-W.; Tan, M. Nanostructures Derived from Starch and Chitosan for Fluorescence Bio-Imaging. Nanomaterials 2016, 6, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Material | TGA | DSC | |
|---|---|---|---|
| Tmax (°C) | ∆trsH (J/g) | Tmax (°C) | |
| CP | 70.6 ± 0.4, 298 ± 1.5 | 200 ± 6.0 | 104 ± 0.3 |
| S | 48.1 ± 0.2, 338 ± 1.7 | 91.4 ± 2.7 | 85.0 ± 0.3 |
| CS-10 | 70.5 ± 0.4, 299 ± 1.5, 347 ± 1.7 | 237 ± 7.1 | 93.3 ± 0.3 |
| CS-30 | 71.7 ± 0.4, 300 ± 1.5, 346 ± 1.7 | 127 ± 3.4 | 84.5 ± 0.3 |
| CS-50 | 70.4 ± 0.4, 300 ± 1.5, 346 ± 1.7 | 119 ± 3.6 | 94.6 ± 0.3 |
| Sample | Tmax (°C) | ΔtrsH (J/g) |
|---|---|---|
| CP-F | 113 ± 0.3 | 1024 ± 31 |
| CP-R | 121 ± 0.4 | 303 ± 9.1 |
| CS-50-F | 105 ± 0.3 | 1840 ± 55 |
| CS-50-R | 101 ± 0.3 | 340 ± 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D. Animal Biopolymer-Plant Biomass Composites: Synergism and Improved Sorption Efficiency. J. Compos. Sci. 2020, 4, 15. https://doi.org/10.3390/jcs4010015
Mohamed MH, Udoetok IA, Wilson LD. Animal Biopolymer-Plant Biomass Composites: Synergism and Improved Sorption Efficiency. Journal of Composites Science. 2020; 4(1):15. https://doi.org/10.3390/jcs4010015
Chicago/Turabian StyleMohamed, Mohamed H., Inimfon A. Udoetok, and Lee D. Wilson. 2020. "Animal Biopolymer-Plant Biomass Composites: Synergism and Improved Sorption Efficiency" Journal of Composites Science 4, no. 1: 15. https://doi.org/10.3390/jcs4010015
APA StyleMohamed, M. H., Udoetok, I. A., & Wilson, L. D. (2020). Animal Biopolymer-Plant Biomass Composites: Synergism and Improved Sorption Efficiency. Journal of Composites Science, 4(1), 15. https://doi.org/10.3390/jcs4010015