Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Extraction of Chitosan from Waste Prawn Shell
2.2.2. Purification and Modification of Bijoypur Clay
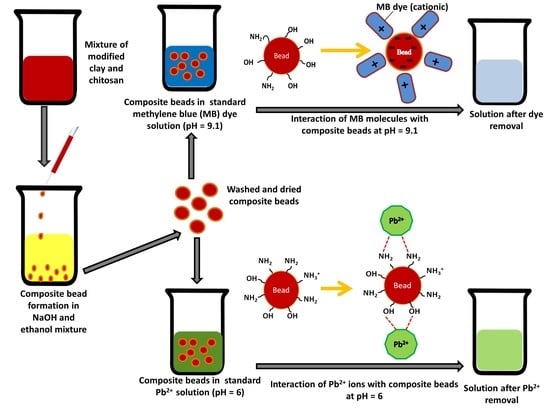
2.2.3. Fabrication of Biocomposite Beads
2.2.4. Characterization of Biocomposite Beads
2.2.5. Adsorption Studies for Standard Solution and Industrial Effluents
3. Results
3.1. Characterization of Biocomposite Beads
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
3.1.3. SEM Analysis
3.2. Comparative Adsorption Studies on a Standard Solution
3.3. Preliminary Application of the Adsorbent in the Industrial Effluent
3.3.1. Removal of Cr (VI) and Pb (II) from Tannery Effluent
3.3.2. Removal of Methylene Blue Dye from Textile Effluent
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balaji, V.; Datta, S.; Bhattacharjee, C. Evaluation on biological treatment for industrial wastewater. Biomed. Environ. Sci. 2005, 85, 320–326. [Google Scholar]
- Kabir, S.; Rashid, T.U.; Negulescu, I.I. Gelation of Textile Dye Solution Treated with Fish Scales. Gels 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.M.; Khan, M.N.; Biswas, S.; Choudhury, T.R.; Haque, P.; Rashid, T.U.; Rahman, M.M. Preparation and characterization of bijoypur clay-crystalline cellulose composite for application as an adsorbent. Adv. Mater. Sci 2017, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Luna, I.Z.; Islam, M.M.; Sharmeen, S.; Salem, K.S.; Rashid, T.U.; Zaman, A.; Haque, P.; Rahman, M.M. Cellulase in Waste Management Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 237–256. [Google Scholar]
- Amin, M.; Dey, S.C.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. Solar assisted photocatalytic degradation of reactive azo dyes in presence of anatase titanium dioxide. Int. J. Latest Res. Eng. Technol. 2016, 2, 14–21. [Google Scholar]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- García-García, A.; Martínez-Miranda, V.; Martínez-Cienfuegos, I.G.; Almazán-Sánchez, P.T.; Castañeda-Juárez, M.; Linares-Hernández, I. Industrial wastewater treatment by electrocoagulation–electrooxidation processes powered by solar cells. Fuel 2015, 149, 46–54. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Bee, A.; Obeid, L.; Mbolantenaina, R.; Welschbillig, M.; Talbot, D. Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 2017, 421, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment. In Green Adsorbents for Pollutant Removal; Springer: Cham, Switzerland, 2018; pp. 23–71. [Google Scholar]
- Leal, T.W.; Lourenço, L.A.; Scheibe, A.S.; de Souza, S.M.G.U.; de Souza, A.A.U. Textile wastewater treatment using low-cost adsorbent aiming the water reuse in dyeing process. J. Environ. Chem. Eng. 2018, 6, 2705–2712. [Google Scholar] [CrossRef]
- Shahzad, A. (Ed.) Green biocomposites from renewable biopolymers and their biomedical applications. In Biocomposites: Properties, Performance and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 473–541. [Google Scholar]
- Crini, G.; Lichtfouse, E. Green Adsorbents for Pollutant Removal: Fundamentals and Design; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Desbrieres, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rashid, T.U.; Datta, A. Chitosan: Process and Modification. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: London, UK, 2015; Volume 11, pp. 1811–1825. [Google Scholar]
- Rashid, T.U.; Islam, M.S.; Sharmeen, S.; Biswas, S.; Zaman, A.; Khan, M.N.; Mallik, A.K.; Haque, P.; Rahman, M.M. Applications of Chitosan Derivatives in Wastewater Treatment. In Handbook of Composites from Renewable Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 471–517. [Google Scholar]
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Sultan, M.; Ashaduzzaman, M.; Sarker, M.; Shamsuddin, S. Preparation, characterization and performance evaluation of chitosan as an adsorbent for remazol red. Int. J. Latest Res. Eng. Technol. 2016, 2, 52–62. [Google Scholar]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Kabir, S.; Rashid, T.U.; Nesa, B.; Nasrin, R.; Haque, P.; Khan, M.A. Effect of γ-irradiation on the thermomechanical and morphological properties of chitosan obtained from prawn shell: Evaluation of potential for irradiated chitosan as plant growth stimulator for Malabar spinach. Radiat. Phys. Chem. 2013, 82, 112–118. [Google Scholar] [CrossRef]
- Bayón, B.; Berti, I.R.; Gagneten, A.M.; Castro, G.R. Biopolymers from Wastes to High-Value Products in Biomedicine. In Waste to Wealth; Springer: Singapore, 2018; pp. 1–44. [Google Scholar]
- Islam, M.S.; Haque, P.; Rashid, T.U.; Khan, M.N.; Mallik, A.K.; Khan, M.N.I.; Khan, M.; Rahman, M.M. Core-shell drug carrier from folate conjugated chitosan obtained from prawn shell for targeted doxorubicin delivery. J. Mater. Sci. Mater. Med. 2017, 28, 55. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Biswas, S.; Rashid, T.U.; Mallik, A.K.; Islam, M.; Khan, M.N.; Haque, P.; Khan, M.; Rahman, M.M. Facile Preparation of Biocomposite from Prawn Shell Derived Chitosan and Kaolinite-Rich Locally Available Clay. Int. J. Polym. Sci. 2017, 6472131. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Karathanasis, A.D. Clay Minerals: Weathering and Alteration of Encyclopedia of Soil Science; CRC Press: Boca Raton, FL, USA, 2017; pp. 430–434. [Google Scholar]
- Oun, A.; Tahri, N.; Mahouche-Chergui, S.; Carbonnier, B.; Majumdar, S.; Sarkar, S.; Sahoo, G.C.; Amar, R.B. Tubular ultrafiltration ceramic membrane based on titania nanoparticles immobilized on macroporous clay-alumina support: Elaboration, characterization and application to dye removal. Sep. Purif. Technol. 2017, 188, 126–133. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Abedi, B.; Bodaghi, H.; Davarynejad, G.; Haratizadeh, H.; Conte, A. Investigation of developed clay-nanocomposite packaging film on quality of peach fruit (Prunus persica Cv. Alberta) during cold storage. J. Food Process. Preserv. 2018, 42, e13466. [Google Scholar] [CrossRef]
- Martino, L.; Guigo, N.L.; van Berkel, J.G.L.; Sbirrazzuoli, N. Influence of organically modified montmorillonite and sepiolite clays on the physical properties of bio-based poly (ethylene 2,5-furandicarboxylate). Compos. Part B Eng. 2017, 110, 96–105. [Google Scholar] [CrossRef]
- Biswas, S.; Islam, M.M.; Hasan, M.; Rimu, S.; Khan, M.; Haque, P.; Rahman, M. Evaluation of Cr (VI) Ion Removal from Aqueous Solution by Bio-Inspired Chitosan-Clay Composite: Kinetics and Isotherms. Iran. J. Chem. Eng. 2018, 15, 63–80. [Google Scholar]
- Rashid, T.U.; Rahman, M.M.; Kabir, S.; Shamsuddin, S.M.; Khan, M.A. A new approach for the preparation of chitosan from γ-irradiation of prawn shell: Effects of radiation on the characteristics of chitosan. Polym. Int. 2012, 61, 1302–1308. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and properties of polyimide–clay hybrid. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- American Public Health; American Water Works; Water Pollution Control; Water Environment. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1915. [Google Scholar]
- Deepali, K.K.; Gangwar, K. Metals concentration in textile and tannery effluents, associated soils and ground water. N. Y. Sci. J. 2010, 3, 82–89. [Google Scholar]
- Li, Y.; Jin, Z.; Li, T.; Li, S. Removal of hexavalent chromium in soil and groundwater by supported nano zero-valent iron on silica fume. Water Sci. Technol. 2011, 63, 2781–2787. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Influence of pH on Cr (VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012010. [Google Scholar]
- Pandey, S.; Mishra, S.B. Organic–inorganic hybrid of chitosan/organoclay bionanocomposites for hexavalent chromium uptake. J. Colloid Interface Sci. 2011, 361, 509–520. [Google Scholar] [CrossRef]
- Jin, Q.; Li, Y.; Yang, D.; Cui, J. Chitosan-derived three-dimensional porous carbon for fast removal of methylene blue from wastewater. RSC Adv. 2018, 8, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Badmus, M.; Audu, T.; Anyata, B. Removal of lead ion from industrial wastewaters by activated carbon prepared from periwinkle shells (Typanotonus fuscatus). Turk. J. Eng. Environ. Sci. 2007, 31, 251–263. [Google Scholar]
- Tsai, W.-C.; Ibarra-Buscano, S.; Kan, C.-C.; Futalan, C.M.; Dalida, M.L.P.; Wan, M.-W. Removal of copper, nickel, lead, and zinc using chitosan-coated montmorillonite beads in single-and multi-metal system. Desalin. Water Treat. 2016, 57, 9799–9812. [Google Scholar] [CrossRef]
- Anwar, Y. Antibacterial and lead ions adsorption characteristics of chitosan-manganese dioxide bionanocomposite. Int. J. Biol. Macromol. 2018, 111, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Huang, W.; Li, Z. Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int. J. Biol. Macromol. 2019, 136, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]









| Biocomposite (by Weight) | Sample ID | CHT (% wt.) in 1% Acetic Solution | MC (% wt.) in 1% Acetic Solution |
|---|---|---|---|
| CHT-MC (2:1) | SB-1 | 1.50 | 0.33 |
| CHT-MC (1:1) | SB-3 | 0.50 | 0.50 |
| CHT-MC (1:2) | SB-5 | 0.33 | 1.50 |
| Adsorbate | Adsorption Capacities for Adsorbents (mg/g) | Best Adsorbent | |||||
|---|---|---|---|---|---|---|---|
| Chitosan | Raw Clay | Modified Clay | SB-1 | SB-3 | SB-5 | ||
| Cr (VI) * | 3.98 | 2.23 | 3.45 | 73 | 64 | 49 | SB-1 [30] |
| MB | 1.01 | 1.30 | 1.83 | 1.682 | 1.95 | 2.385 | SB-5 |
| Pb (II) | 5.184 | 2.632 | 3.86 | 5.55 | 5.052 | 4.032 | SB-1 |
| Contaminants | Effluents | Composites | Concentration (ppm) | Removal (%) ((Cb − Ca)/Cb) × 100% | |
|---|---|---|---|---|---|
| Before (Cb) | After (Ca) | ||||
| Cr (VI) | Tannery | SB-1 | 0.110 | 0.054 | 50.90 |
| Pb (II) | Tannery | SB-1 | 0.081 | 0.049 | 39.50 |
| MB | Textile | SB-5 | 0.190 | 0.130 | 31.50 |
| Contaminants | Composites | Concentration (ppm) | Removal (%) ((Cb − Ca)/Cb) × 100% | |
|---|---|---|---|---|
| Before (Cb) | After (Ca) | |||
| Cr (VI) | SB-1 | 25 | 5.21 | 67.16 |
| Pb (II) | SB-1 | 25 | 11.13 | 55.48 |
| MB | SB-5 | 25 | 14.85 | 40.60 |
| Metal/Dye | Adsorbents | Source | Initial Concentration (mg/L) | Maximum Sorption Capacity Qmax (mg/g) | Percentage Removal (%) | Ref. |
|---|---|---|---|---|---|---|
| Pb (II) | Chitosan-coated montmorillonite | Groundwater | 3 | - | 94.08 | [41] |
| Activated periwinkle shell carbon | Effluent (pH 8.7) | 19.1 | - | 82.78 | [40] | |
| Commercial activated carbon | Effluent (pH 8.7) | 19.1 | - | 92.68 | ||
| Chitosan | Aqueous solution | 100 | - | 50.30 | [42] | |
| Chitosan–manganese dioxide | Aqueous solution | 100 | - | 88.70 | ||
| Chitosan–kaolinite rich modified clay beads | Aqueous standard solution | 25 | - | 55.48 * | Present study | |
| Tannery effluent | 0.081 | - | 39.50 ** | |||
| MB | Chitosan cross-linked graphene oxide/lignosulfonate composite | Aqueous solution | 100 | 1023.9 | >99% | [43] |
| Chitosan-derived three-dimensional porous carbon | Wastewater | - | 925.93 | >93.40 | [39] | |
| Activated carbon from apricot stones | Aqueous solution | 10 | - | 99.5 | [44] | |
| Chitosan-kaoline rich modified clay beads | Standard solution | 25 | - | 40.60 * | Present study | |
| Textile effluent | 0.190 | - | 31.50 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.; Rashid, T.U.; Debnath, T.; Haque, P.; Rahman, M.M. Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. J. Compos. Sci. 2020, 4, 16. https://doi.org/10.3390/jcs4010016
Biswas S, Rashid TU, Debnath T, Haque P, Rahman MM. Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. Journal of Composites Science. 2020; 4(1):16. https://doi.org/10.3390/jcs4010016
Chicago/Turabian StyleBiswas, Shanta, Taslim Ur Rashid, Tonmoy Debnath, Papia Haque, and Mohammed Mizanur Rahman. 2020. "Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent" Journal of Composites Science 4, no. 1: 16. https://doi.org/10.3390/jcs4010016
APA StyleBiswas, S., Rashid, T. U., Debnath, T., Haque, P., & Rahman, M. M. (2020). Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. Journal of Composites Science, 4(1), 16. https://doi.org/10.3390/jcs4010016