Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
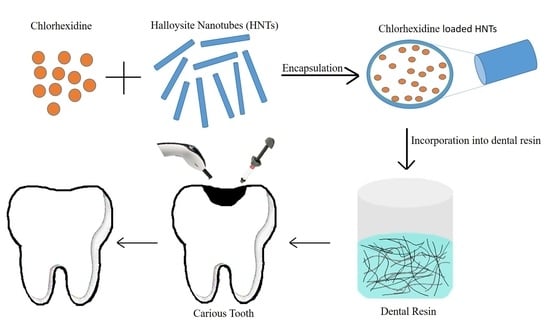
2.2.1. Drug Loading into HNTs
2.2.2. Preparation of Dental Resin Composites
2.3. Characterization
2.3.1. Characterization of Inorganic Fillers
2.3.2. Characterization of Dental Resin Composites
2.3.3. Statistical Analysis
3. Results
3.1. Morphological Evaluation
3.2. Diffraction Studies of HNT, CHX and HNT/CHX
3.3. FTIR Analysis of HNT, CHX and HNT/CHX
3.4. Properties of Dental Resin Composites
3.4.1. Mechanical Characterization
3.4.2. Degree of Conversion
3.4.3. Curing Depth
3.4.4. Morphological Studies of Fractured Dental Resin Composites
3.4.5. Antimicrobial Activity
3.4.6. In Vitro Cytotoxicity Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease is there cause for consideration. Microorganisms 2019, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Frankenberger, R.; Pashley, D.H.; Reich, S.M.; Lohbauer, U.; Petschelt, A.; Tay, F.R. Characterisation of resin–dentine interfaces by compressive cyclic loading. Biomaterials 2005, 26, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Palasuk, J.; Windsor, L.J.; Platt, J.A.; Lvov, Y.; Geraldeli, S.; Bottino, M.C. Doxycycline-loaded nanotube-modified adhesives inhibit MMP in a dose-dependent fashion. Clin. Oral Investig. 2018, 22, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Pashley, D.H.; Marchesi, G.; Carrilho, M.; Antoniolli, F.; Mazzoni, A.; Tay, F.R.; Di Lenarda, R.; Breschi, L. Influence of chlorhexidine on the degree of conversion and E-modulus of experimental adhesive blends. Dent. Mater. 2009, 25, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, 41–53. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mjör, I.A. The reasons for replacement and the age of failed restorations in general dental practice. Acta Odontol. Scand. 1997, 55, 58–63. [Google Scholar] [CrossRef]
- Khvostenko, D.; Salehi, S.; Naleway, S.E.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent. Mater. 2015, 31, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Rawtani, D.; Agrawal, Y.K. Multifarious applications of halloysite nanotubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 14, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes in vivo: A Caenorhabditiselegans study. Environ. Sci. 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Salim, N.; Moore, C.; Silikas, N.; Satterthwaite, J.; Rautemaa, R. Chlorhexidine is a highly effective topical broad-spectrum agent against Candida spp. Int. J. Antimicrob. Agents 2013, 41, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Basrani, B. Chlorhexidinegluconate. Aust. Endod. J. 2005, 31, 48–52. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Zheng, F.; Liu, H.; Yu, J.; Shi, X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly (lactic-co-glycolic acid) composite nanofibers. Colloids Surf. B Biointerfaces 2013, 110, 148–155. [Google Scholar] [CrossRef]
- Zandinejad, A.A.; Atai, M.; Pahlevan, A. The effect of ceramic and porous fillers on the mechanical properties of experimental dental composites. Dent. Mater. 2006, 22, 382–387. [Google Scholar] [CrossRef]
- Wegehaupt, F.J.; Lunghi, N.; Belibasakis, G.N.; Attin, T. Influence of light-curing distance on degree of conversion and cytotoxicity of etch-and-rinse and self-etch adhesives. BMC Oral Health 2017, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Rawtani, D.; Pandey, G.; Tharmavaram, M.; Pathak, P.; Akkireddy, S.; Agrawal, Y.K. Development of a novel ‘nanocarrier’system based on Halloysite Nanotubes to overcome the complexation of ciprofloxacin with iron: An in vitro approach. Appl. Clay Sci. 2017, 150, 293–302. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P.; Hussain, C.M.; Akkireddy, S. Physicochemical and biological assessment of flowable resin composites incorporated with farnesol loaded halloysite nanotubes for dental applications. J. Mech. Behav. Biomed. Mater. 2020, 7, 103675. [Google Scholar] [CrossRef]
- Chen, H.; Wang, R.; Zhang, J.; Hua, H.; Zhu, M. Synthesis of core-shell structured ZnO@ m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent. Mater. 2018, 34, 1846–1855. [Google Scholar] [CrossRef]
- TÜRKÜN, L.S.; TÜRKÜN, M.; ERTUG˘ RUL, F.A.; Ates, M.; Brugger, S. Long-term antibacterial effects and physical properties of a chlorhexidine-containing glass ionomer cement. J. Esthet. Restor. Dent. 2008, 20, 29–44. [Google Scholar] [CrossRef]
- Imazato, S.; Imai, T.; Russell, R.R.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 39, 511–515. [Google Scholar] [CrossRef]
- Hotwani, K.; Thosar, N.; Baliga, S.; Bundale, S.; Sharma, K. Antibacterial effects of hybrid tooth colored restorative materials against Streptococcus mutans: An in vitro analysis. J. Conserv. Dent. 2013, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Tumscitz, D.B.; Laxe, L.A.; Pascoal, A.C.; HIRATA JUNIOR, R.; Lins, R.X. Cytotoxicity of three light-cured resin cements on 3T3 fibroblasts. Rev Fac Odontol Pernambuco. 2017, 46, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, P.; Rawtani, D.; Barot, T. Formulation and optimization of long acting dual niosomes using Box-Behnken experimental design method for combinative delivery of Ethionamide and D-cycloserine in Tuberculosis treatment. Colloids Surf. Physicochem. Eng. Asp. A 2019, 565, 131–142. [Google Scholar] [CrossRef]
- De Silva, R.T.; Dissanayake, R.K.; Mantilaka, M.P.; Wijesinghe, W.S.; Kaleel, S.S.; Premachandra, T.N.; Weerasinghe, L.; Amaratunga, G.A.; De Silva, K.N. Drug-Loaded Halloysite Nanotube-Reinforced Electrospun Alginate-Based Nanofibrous Scaffolds with Sustained Antimicrobial Protection. ACS Appl. Mater. Interfaces 2018, 10, 33913–33922. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.M.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Guo, B.; Jia, D. Thermal stability and flame retardant effects of halloysite nanotubes on poly (propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Liu, H.; Yao, X.; Leng, F.; Chen, Y.; Tian, W. Long-term antibacterial protected cotton fabric coating by controlled release of chlorhexidine gluconate from halloysite nanotubes. Rsc Adv. 2017, 7, 18917–18925. [Google Scholar] [CrossRef] [Green Version]
- Levis, S.R.; Deasy, P.B. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002, 243, 125–134. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, J.H.; Ryu, H.J.; Elzatahry, A.A.; Alothman, Z.A.; Choy, J.H. Drug–clay nanohybrids as sustained delivery systems. Appl. Clay Sci. 2016, 130, 20–32. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Wu, W.; Xu, T.; Fong, H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing halloysite nanotubes. Dent. Mater. 2012, 28, 1071–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottino, M.C.; Batarseh, G.; Palasuk, J.; Alkatheeri, M.S.; Windsor, L.J.; Platt, J.A. Nanotube-modified dentin adhesive—Physicochemical and dentin bonding characterizations. Dent. Mater. 2013, 29, 1158–1165. [Google Scholar] [CrossRef]
- CHUNG, K.H.; Greener, E.H. Correlation between degree of conversion, filler concentration and mechanical properties of posterior composite resins. J. Oral Rehabil. 1990, 17, 487–494. [Google Scholar] [CrossRef]
- Eldiwany, M.; Powers, J.M.; George, L.A. Mechanical properties of direct and post-cured composites. Am. J. Dent. 1993, 6, 222–224. [Google Scholar]
- Lovell, L.G.; Lu, H.; Elliott, J.E.; Stansbury, J.W.; Bowman, C.N. The effect of cure rate on the mechanical properties of dental resins. Dent. Mater. 2001, 17, 504–511. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.N.; Bhowmick, A.K.; Heinrich, G. Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Lauruengtana, V.; Surassmo, S.; Ruktanonchai, U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 240–249. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariga, K.; Lvov, Y.M.; Kawakami, K.; Ji, Q.; Hill, J.P. Layer-by-layer self-assembled shells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay nanotube encapsulation for functional biocomposites. Adv. Colloid Interface Sci. 2014, 207, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Cenci, T.; Cenci, M.S.; Fedorowicz, Z.; Azevedo, M. Antibacterial agents in composite restorations for the prevention of dental caries. Cochrane Database Syst. Rev. 2013, 12. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–2903. [Google Scholar] [CrossRef]
- Miao, X.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H. Low shrinkage light curable dental nanocomposites using SiO2 microspheres as fillers. Mater. Sci. Eng. C 2012, 32, 2115–2121. [Google Scholar] [CrossRef]








| Groups | % wt. of HNT/CHX | Resin (Matrix) | Other Component | Light-Curing Time (s) | No. of Samples |
|---|---|---|---|---|---|
| H1 (Control) | 0 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| H2 | 1 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| H3 | 2.5 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| H4 | 5 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| H5 | 7.5 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| H6 | 10 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| Groups | % wt. of HNT/CHX | % wt. of Glass Fillers | Resin (Matrix) | Other Component | Light-Curing Time (s) | No. of Samples |
|---|---|---|---|---|---|---|
| G1 (Control) | 0 | 70 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| G2 | 1 | 69 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| G3 | 2.5 | 67.5 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| G4 | 5 | 65 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| G5 | 7.5 | 62.5 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
| G6 | 10 | 60 | Bis-GMA/TEGDMA 69.5/29.5 wt.% | CQ a/4-EDMAB b 0.5/0.5 wt.% | 40 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barot, T.; Rawtani, D.; Kulkarni, P. Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications. J. Compos. Sci. 2020, 4, 81. https://doi.org/10.3390/jcs4020081
Barot T, Rawtani D, Kulkarni P. Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications. Journal of Composites Science. 2020; 4(2):81. https://doi.org/10.3390/jcs4020081
Chicago/Turabian StyleBarot, Tejas, Deepak Rawtani, and Pratik Kulkarni. 2020. "Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications" Journal of Composites Science 4, no. 2: 81. https://doi.org/10.3390/jcs4020081
APA StyleBarot, T., Rawtani, D., & Kulkarni, P. (2020). Development of Chlorhexidine Loaded Halloysite Nanotube Based Experimental Resin Composite with Enhanced Physico-Mechanical and Biological Properties for Dental Applications. Journal of Composites Science, 4(2), 81. https://doi.org/10.3390/jcs4020081