Stability Enhancement of Laser-Scribed Reduced Graphene Oxide Electrodes Functionalized by Iron Oxide/Reduced Graphene Oxide Nanocomposites for Nitrite Sensors
Abstract
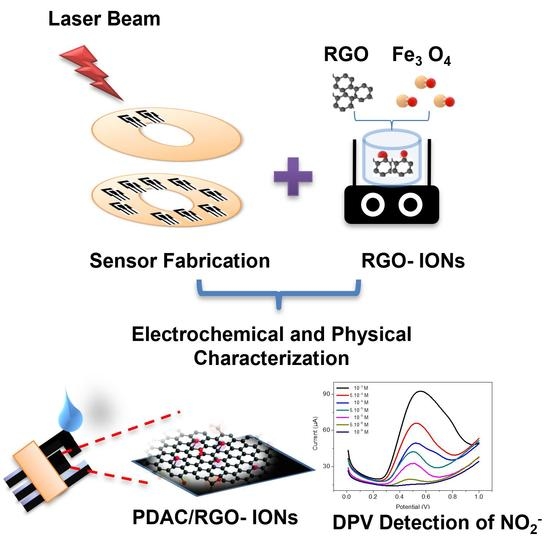
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Device Fabrication and Microstructural Analysis
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Structural and Morphological Characterization of ION-RGO Nanocomposite, LRGO, and LRGO/PDAC/ION-RGO Electrodes
3.2. Electrochemical Properties of LRGO Electrodes
3.3. Electrochemical Application: Oxidation of Nitrite
3.4. Performance of LRGO/PDAC/ION-RGO Electrode in the Voltammetric Determination of Nitrite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, J.O. Nitrate- and Nitrite-Accumulating Plants. In Veterinary Toxicology: Basic and Clinical Principles: Third Edition; Elsevier: Amsterdam, The Netherlands, 2018; pp. 941–946. ISBN 9780128114100. [Google Scholar]
- Pooja, D.; Kumar, P.; Singh, P. Sensors in Water Pollutants Monitoring: Role of Material Advanced Functional Materials and Sensors; Springer: Singapore, 2020; p. 320. [Google Scholar]
- Moo, Y.C.; Matjafri, M.Z.; Lim, H.S.; Tan, C.H. New Development of Optical Fibre Sensor for Determination of Nitrate and Nitrite in Water. Optik 2016, 127, 1312–1319. [Google Scholar] [CrossRef]
- Hutton, G.; Haller, L. Evaluation of the Costs and Benefits of Water and Sanitation Improvements at the Global Level; World Health Organization: Gevena, Switzerland, 1994. [Google Scholar]
- Tsikas, D. ReviewMethods of Quantitative Analysis of the Nitric Oxide Metabolites Nitrite and Nitrate in Human Biological Fluids Methods of Quantitative Analysis of the Nitric Oxide Metabolites Nitrite and Nitrate in Human Biological Fluids. Free. Radic. Res. 2009, 39, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Thipwimonmas, Y.; Jaidam, J.; Samoson, K.; Khunseeraksa, V.; Phonchai, A.; Thiangchanya, A.; Haw Chang, K.; Fahmi Lim Abdullah, A.; Limbut, W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample. Chemosensors 2021, 9, 161. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Islam, A.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Introducing an Amine Functionalized Novel Conjugate Material for Toxic Nitrite Detection and Adsorption from Wastewater. J. Clean. Prod. 2019, 228, 778–785. [Google Scholar] [CrossRef]
- Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ali, B.; et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring Academic Editors: Najla Fourati And. Sensors 2021, 21, 4131. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-Imprinted Electrochemical Sensor Based on Copper Nanoparticles-Polyaniline Matrix for Nitrate Detection. J. Sens. 2019, 2019, 4257125. [Google Scholar] [CrossRef]
- Tan, J.F.; Anastasi, A.; Chandra, S. Electrochemical Detection of Nitrate, Nitrite and Ammonium for on-Site Water Quality Monitoring. Curr. Opin. Electrochem. 2022, 32, 100926. [Google Scholar] [CrossRef]
- Mao, Y.; Bao, Y.; Han, D.X.; Zhao, B. Research Progress on Nitrite Electrochemical Sensor. Chin. J. Anal. Chem. 2018, 46, 147–155. [Google Scholar] [CrossRef]
- Zou, H.L.; Qin, L.Y.; Luo, H.Q.; Li, B.L.; Li, N.B. High-Valence Mo(VI) Derived from in-Situ Oxidized MoS2 Nanosheets Enables Enhanced Electrochemical Responses for Nitrite Measurements. Sens. Actuators B Chem. 2021, 337, 129812. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water-A Review. Sensors 2019, 20, 54. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Waseem Akram, M.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Talbi, M.; Al-Hamry, A.; Rios Teixeira, P.; Paterno, L.G.; Ali, B.; Kanoun, O. Enhanced Nitrite Detection by a Carbon Screen Printed Electrode Modified with Photochemically-Made AuNPs. Chemosensors 2022, 10, 40. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; ben Ali, M.; Kanoun, O. Electrochemical Sensor for Nitrite Detection in Water Samples Using Flexible Laser-Induced Graphene Electrodes Functionalized by CNT Decorated by Au Nanoparticles. J. Electroanal. Chem. 2021, 880, 114893. [Google Scholar] [CrossRef]
- Al-Hamry, A.; Kang, H.; Sowade, E.; Dzhagan, V.; Rodriguez, R.D.; Müller, C.; Zahn, D.R.T.; Baumann, R.R.; Kanoun, O. Tuning the Reduction and Conductivity of Solution-Processed Graphene Oxide by Intense Pulsed Light. Carbon 2016, 102, 236–244. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental Review: Chemical Reduction of Graphene Oxide (GO) to Reduced Graphene Oxide (RGO) by Aqueous Chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef]
- Choi, S.; Kim, C.; Suh, J.M.; Jang, H.W. Reduced Graphene Oxide-Based Materials for Electrochemical Energy Conversion Reactions. Carbon Energy 2019, 1, 85–101. [Google Scholar] [CrossRef]
- Lin, S.; Feng, W.; Miao, X.; Zhang, X.; Chen, S.; Chen, Y.; Wang, W.; Zhang, Y. A Flexible and Highly Sensitive Nonenzymatic Glucose Sensor Based on DVD-Laser Scribed Graphene Substrate. Biosens. Bioelectron. 2018, 110, 89–96. [Google Scholar] [CrossRef]
- Aparicio-Martínez, E.; Ibarra, A.; Estrada-Moreno, I.A.; Osuna, V.; Dominguez, R.B. Flexible Electrochemical Sensor Based on Laser Scribed Graphene/Ag Nanoparticles for Non-Enzymatic Hydrogen Peroxide Detection. Sens. Actuators B Chem. 2019, 301, 127101. [Google Scholar] [CrossRef]
- Strong, V.; Dubin, S.; El-Kady, M.F.; Lech, A.; Wang, Y.; Weiller, B.H.; Kaner, R.B. Patterning and Electronic Tuning of Laser Scribed Graphene for Flexible All-Carbon Devices. ACS Nano 2012, 6, 1395–1403. [Google Scholar] [CrossRef]
- Brahem, A.; Al-Hamry, A.; Reddy, A.; Bouhamed, A.; Nasrallah, S.B.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Elaboration of Reduced Graphene Oxide Electrodes Functionalized with RGO-ION Composite for Nitrite Detection in Water. In Proceedings of the GMA/ITG-Fachtagung Sensoren und Messsysteme 2019, Nürnberg, Germany, 25–26 June 2019. [Google Scholar]
- Brahem, A.; Al-Hamry, A.; ben Ali, M.; Kanoun, O. Stability Investigation of Electrochemical Laser Scribed RGO Sensors for Nitrite Detection. In Proceedings of the 2019 5th International Conference on Nanotechnology for Instrumentation and Measurement, NanofIM 2019, Institute of Electrical and Electronics Engineers Inc., Piscataway Township, NJ, USA, 30 October 2019. [Google Scholar]
- Peregrino, P.P.; Cavallari, M.R.; Fonseca, F.J.; Moreira, S.G.C.; Sales, M.J.A.; Paterno, L.G. Starch-Mediated Immobilization, Photochemical Reduction, and Gas Sensitivity of Graphene Oxide Films. ACS Omega 2020, 5, 5001–5012. [Google Scholar] [CrossRef]
- Teo, P.S.; Lim, H.N.; Huang, N.M.; Chia, C.H.; Harrison, I. Room Temperature in Situ Chemical Synthesis of Fe3O4/Graphene. Ceram. Int. 2012, 38, 6411–6416. [Google Scholar] [CrossRef]
- Gonzálezgonz´gonzález-Alonso, D.; Espeso, J.I.; Gavií, H.; Zeng, L.J.; Fernández, M.T.; Fernández-Díaz, F.; Subíassubías, G.; de Pedro, I.; Rodríguez, J.; Fernández, R.; et al. Identifying the Presence of Magnetite in an Ensemble of Iron-Oxide Nanoparticles: A Comparative Neutron Diffraction Study between Bulk and Nanoscale. Nanoscale Adv. 2021, 3, 3491–3496. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.g.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Ghica, C.; Cypriano, J.; Abreu, F.; Anselme, K.; Ersen, O.; Farina, M.; Werckmann, J. New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria. Nanomaterials 2021, 11, 3189. [Google Scholar] [CrossRef] [PubMed]
- Zenou, V.Y.; Bakardjieva, S. Microstructural Analysis of Undoped and Moderately Sc-Doped TiO2 Anatase Nanoparticles Using Scherrer Equation and Debye Function Analysis. Mater. Charact. 2018, 144, 287–296. [Google Scholar] [CrossRef]
- Bogart, L.K.; Fock, J.; da Costa, G.M.; Witte, K.; Greneche, J.M.; Zukrowski, J.; Sikora, M.; Latta, D.E.; Scherer, M.M.; Hansen, M.F.; et al. Prenormative Verification and Validation of a Protocol for Measuring Magnetite–Maghemite Ratios in Magnetic Nanoparticles. Metrologia 2022, 59, 015001. [Google Scholar] [CrossRef]
- Cao, Y.; Mohamed, A.M.; Abdollahzadeh, M.; Huynen, I. Rational Design of CoNi@C-BTC/RGO Nanocomposite Coated with PEDOT Polymer towards Enhancing the Microwave Absorption in X-Band Frequency. J. Alloys Compd. 2021, 862, 158371. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Kim, C.H.; Kim, J.H.; You, I.K.; In, J.b.; Lee, S.M. Fast and Controllable Reduction of Graphene Oxide by Low-Cost CO2 Laser for Supercapacitor Application. Appl. Surf. Sci. 2018, 462, 353–361. [Google Scholar] [CrossRef]
- Ganapathy, R.; Sarmadi, M.; Denes, F. Immobilization of α-Chymotrypsin on Oxygen-RF-Plasma Functionalized PET and PP Surfaces. J. Biomater. Sci. Polym. Ed. 2012, 9, 389–404. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Montazer, M.; Gao, W. Surface Modification of PET Fabric through In-Situ Reduction and Cross-Linking of Graphene Oxide: Towards Developing Durable Conductive Fabric Coatings. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 16–25. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar Diffusion to Macro Disc Electrodes—What Electrode Size Is Required for the Cottrell and Randles-Sevcik Equations to Apply Quantitatively? J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Q.; Li, J.; Hong, C.; Zhao, Z.; Xu, H.; Hu, J. Synthesis and Enhanced Electrochemical Properties of AuNPs@MoS2/RGO Hybrid Structures for Highly Sensitive Nitrite Detection. Microchem. J. 2022, 172, 106904. [Google Scholar] [CrossRef]
- Boussema, F.; Haddad, R.; Ghandour, Y.; Belkhiria, M.S.; Holzinger, M.; Maaref, A.; Cosnier, S. Polyoxometalate [PMo11O39]7−/Carbon Nanocomposites for Sensitive Amperometric Detection of Nitrite. Electrochim. Acta 2016, 222, 402–408. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Pandikumar, A.; Yusoff, N.; Huang, N.M.; Lim, H.N. Electrochemical Sensing of Nitrite Using a Glassy Carbon Electrode Modified with Reduced Functionalized Graphene Oxide Decorated with Flower-like Zinc Oxide. Microchim. Acta 2015, 182, 1113–1122. [Google Scholar] [CrossRef]












| Electrode Material | Detection Method | Linear Range | Working pH | LOD | Reference |
|---|---|---|---|---|---|
| SWCNTs/POM | Amperometry | 30.0–16 × 103 µM | - | 30 µM | [39] |
| GR/ZnO | Amperometry | 10–8.0 × 103 µM | 7.2 | 33 µM | [40] |
| AuNPs-PEI | Voltametric | 0.001−1000 µM | 6.5 | 2.5 nM | [15] |
| f-MWCNT- AuNPs | Voltametric | 10−140 μM | 7 | 0.9 μM | [16] |
| PDAC/ION-RGO | Voltametric | 10–400 µM | 5.7 | 7.2 µM | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahem, A.; Al-Hamry, A.; Gross, M.A.; Paterno, L.G.; Ali, M.B.; Kanoun, O. Stability Enhancement of Laser-Scribed Reduced Graphene Oxide Electrodes Functionalized by Iron Oxide/Reduced Graphene Oxide Nanocomposites for Nitrite Sensors. J. Compos. Sci. 2022, 6, 221. https://doi.org/10.3390/jcs6080221
Brahem A, Al-Hamry A, Gross MA, Paterno LG, Ali MB, Kanoun O. Stability Enhancement of Laser-Scribed Reduced Graphene Oxide Electrodes Functionalized by Iron Oxide/Reduced Graphene Oxide Nanocomposites for Nitrite Sensors. Journal of Composites Science. 2022; 6(8):221. https://doi.org/10.3390/jcs6080221
Chicago/Turabian StyleBrahem, Amina, Ammar Al-Hamry, Marcos Andriola Gross, Leonardo G. Paterno, Mounir Ben Ali, and Olfa Kanoun. 2022. "Stability Enhancement of Laser-Scribed Reduced Graphene Oxide Electrodes Functionalized by Iron Oxide/Reduced Graphene Oxide Nanocomposites for Nitrite Sensors" Journal of Composites Science 6, no. 8: 221. https://doi.org/10.3390/jcs6080221
APA StyleBrahem, A., Al-Hamry, A., Gross, M. A., Paterno, L. G., Ali, M. B., & Kanoun, O. (2022). Stability Enhancement of Laser-Scribed Reduced Graphene Oxide Electrodes Functionalized by Iron Oxide/Reduced Graphene Oxide Nanocomposites for Nitrite Sensors. Journal of Composites Science, 6(8), 221. https://doi.org/10.3390/jcs6080221