An Eco-Friendly, Simple, and Inexpensive Method for Metal-Coating Strontium onto Halloysite Nanotubes
Abstract
:1. Introduction
2. Materials
3. Methods
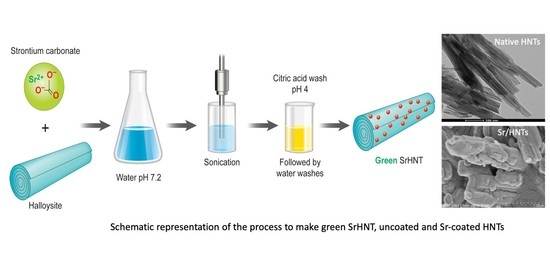
3.1. Preparation of Sr Coated HNTs
3.2. Scanning Electron Microscope (SEM)
3.3. Energy Dispersive Spectroscopy (EDS)
3.4. X-ray Diffraction (XRD)
3.5. Fourier Transformation Infrared (FT-IR) Spectroscopy
3.6. Cell Culture
3.7. Viability and Cytotoxicity Testing
3.8. Cell Proliferation Assay
3.9. Bacterial Growth Rate Study
3.10. Micro Titration Method
3.11. Statistical Analysis
4. Results
4.1. Fabrication Method for Strontium Coating the Halloysite Surface
4.2. Surface Topography
4.3. EDS Analysis
4.4. FTIR-ATR
4.5. XRD Analysis
4.6. Viability and Cytotoxicity Testing for SrHNTs
4.7. Proliferation Assay
4.8. Bacterial Growth Rate
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Sanchez, C.J., Jr.; Ward, C.L.; Romano, D.R.; Hurtgen, B.J.; Hardy, S.K.; Woodbury, R.L.; Trevino, A.V.; Rathbone, C.R.; Wenke, J.C. Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro. BMC Musculoskelet. Disord. 2013, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- De Long, W.G., Jr.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: A critical analysis. J. Bone Jt. Surg. Am. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar] [CrossRef]
- Tan, H.L.; Lin, W.T.; Tang, T.T. The use of antimicrobial impregnated PMMA to manage periprosthetic infections: Controversial issues and the latest developments. Int. J. Artif. Organs. 2012, 35, 832–839. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U.; Mills, D.K. Formulation and Evaluation of Nanoenhanced Antibacterial Calcium Phosphate Bone Cements; Biomaterials, O., Webster, T., Li, B., Eds.; Springer: New York, NY, USA, 2018; pp. 85–108. [Google Scholar]
- Han, S.Y.; Yoon, S.H.; Cho, K.H.; Cho, H.J.; An, J.H.; Ra, Y.S. Biodegradable polymer releasing antibiotic developed for drainage catheter of cerebrospinal fluid: In vitro results. J. Korean Med. Sci. 2005, 20, 297–301. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef]
- Available online: https://www.piedmont.org/spine/conditions-diseases/spine-osteoporosis (accessed on 21 March 2020).
- Knickman, J.R.; Snell, E.K. The 2030 problem: Caring for aging baby boomers. Health Serv. Res. 2002, 37, 849–884. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: A novel mode of action optimizing bone formation and resorption. Osteoporos. Int. 2005, 16, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Ammann, P. Strontium ranelate: A novel mode of action leading to renewed bone quality. Osteoporos. Int. 2005, 16, S11–S15. [Google Scholar] [CrossRef]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium-substituted hydroxyapatite-gelatin biomimetic scaffolds modulate bone cell response. Macromol. Biosci. 2018, 18, e1800096. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Zandieh-Doulabi, B.; Klein-Nulend, J. Strontium ranelate affects signaling from mechanically-stimulated osteocytes towards osteoclasts and osteoblasts. Bone 2013, 53, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.C.; Heiss, G.; Knapp, J.; Thomas, B.; Nies, M.; Gelinsky, D.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef]
- Thormann, U.; Ray, S.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef]
- Agrawal, S.; Kelkar, M.; De, A.; Kulkarni, A.R.; Gandhi, M.N. Surfactant free novel one-minute microwave synthesis, characterization and cell toxicity study of mesoporous strontium hydroxyapatite nanorods. RSC Adv. 2016, 6, 94921–94926. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, P.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+ and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Gazzano, M.; Nervi, C.; Chierotti, M.R.; Rubini, K.; Gobetto, R.; Bigi, A. Strontium and zinc substitution in β-tricalcium phosphate: A d-ray diffraction, solid state NMR and ATR-FTIR study. J. Funct. Biomater. 2019, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Liang, W.; Li, L.; Cui, X.; Wei, X.; Pan, H.; Li, B. Novel calcitonin gene-related peptide/chitosan-strontium-calcium phosphate cement: Enhanced proliferation of human umbilical vein endothelial cells in vitro. J. Biomed. Mat. Res. Pt B Appl. Biomat. 2019, 107, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.V.; Kauppinen, K.; Brooks, R.A.; Best, S.M. An in vitro study into the effect of zinc substituted hydroxyapatite on osteoclast number and activity. J. Biomed. Mater. Res. A 2014, 102, 4136–4141. [Google Scholar] [CrossRef]
- Almeida, M.M.; Nani, E.P.; Teixeira, L.N.; Peruzzo, D.C.; Joly, J.C.; Napimoga, M.H.; Martinez, E.F. Strontium ranelate increases osteoblast activity. Tissue Cell 2016, 48, 183–188. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, A.; Yang, H.; Ouyang, J. Applications and interfaces of halloysite nanocomposites. Appl. Clay Sci. 2015, 119, 8–17. [Google Scholar] [CrossRef]
- Tarasova, E.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Fakhrullin, R. Cytocompatibility and uptake of polycations-modified halloysite clay nanotubes. Appl. Clay Sci. 2015, 119, 21–30. [Google Scholar] [CrossRef]
- Abdllayev, E.; Lvov, Y. Functional polymer clay nanotube composites with sustained release of chemical agents. Prog. Poly. Sci. 2013, 38, 1690–1719. [Google Scholar]
- Deng, S.; Zhang, J.; Ye, L. Halloysite-epoxy nanocomposites with improved particle dispersion through ball mill homogenisation and chemical treatments. Compos. Sci. Technol. 2009, 69, 2497–2505. [Google Scholar] [CrossRef]
- Wei, W.; Abdullayev, E.; Hollister, A.; Mills, D.; Lvov, Y.M. Clay nanotube/poly(methyl methacrylate) bone cement composites with sustained antibiotic release. Macromol. Mater. Eng. 2012, 297, 645–653. [Google Scholar] [CrossRef]
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Yuri Lvov, and Anshul Agarwal. Halloysite clay nanotubes for life sciences applications: From drug encapsulation to bioscaffold. Adv. Colloid Interface Sci. 2018, 257, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 389. [Google Scholar] [CrossRef] [PubMed]
- Yue Li, Y.; Mills, D.K. Halloysite nanotubes as a potential chemotactic agent for bone repair. In Proceedings of the Orthopedic Research Society Meeting, Phoenix, Arizona, 8–11 February 2020. [Google Scholar]
- Tappa, K.; Jammalamadaka, U.; Mills, D.K. Formulation and evaluation of nanoenhanced antibacterial calcium phosphate bone cements. In Orthopedic Biomaterials; Springer: Cham, Switzerland, 2017; pp. 85–108. [Google Scholar]
- Jammalamadaka, U.; Tappa, K.; Mills, D. Osteoinductive calcium phosphate clay nanoparticle bone cements (CPCs) with enhanced mechanical properties. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3921–3924. [Google Scholar]
- Karnik, S.; Mills, D.K. Nanoenhanced hydrogel system with sustained release capabilities. J. Biomed. Mater. Res. Part A 2015, 103, 2416–2426. [Google Scholar] [CrossRef]
- Karnik, S.; Jammalaka, U.; Tappa, K.; Mills, D.K. Performance evaluation of nanoclay enriched antimicrobial hydrogels for biomedical applications. Heliyon 2016, 2, e00072. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.C.; Weisman, J.A.; Boyer, C.J.; Wilson, C.G.; Mills, D.K. Dry sintered metal coating of halloysite nanotubes. Appl. Sci. 2016, 6, 265. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite nanotubes supported Ag and ZnO nanoparticles with synergistically enhanced antibacterial activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Ouyang, J.; Yang, H. Palladium nanoparticles deposited on silanized halloysite nanotubes: Synthesis, characterization and enhanced catalytic property. Sci. Rep. 2013, 3, 2948. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Zhang, Y.; Zhang, D.; Zhu, J. Effect of preparation method on halloysite supported cobalt catalysts for Fischer-Tropsch synthesis. J. Nat. Gas Chem. 2012, 21, 426–430. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, M. Silver nanoparticle supported on halloysite nanotubes catalyzed reduction of 4-nitrophenol (4-NP). Appl. Surf. Sci. 2009, 255, 3989–3993. [Google Scholar] [CrossRef]
- Abdullayev, E.; Sakakibara, K.; Okamoto, K.; Wei, W.; Ariga, K.; Lvov, Y. Natural tubule clay template synthesis of silver nanorods for antibacterial composite coating. Am. Chem. Soc. Appl. Mater. Interfaces 2011, 3, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, L.; Shen, B.; Wang, C. Halloysite-nanotubes supported FeNi alloy nanoparticles for catalytic decomposition of toxic phosphine gas into yellow phosphorous and hydrogen. Chemosphere 2013, 91, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Ivanov, E.V.; Shrestha, L.K.; Ariga, K.; Darrat, Y.A.; Lvov, Y.M. Formation of metal clusters in halloysite clay nanotubes. Sci. Technol. Adv. Mater. 2017, 18, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Note, R.; Riela, S.J. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Markowska, K.; Grudniak, A.M.; Wolska, K.I. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013, 60, 523–530. [Google Scholar] [CrossRef]
- Rezazadeh, T.; Khan, M.D.; Riche, K.; Lvov, Y.M.; Stavisky, A.V.; Wiley, J.B. Rapid and controlled in situ growth of noble metal nanostructures within halloysite clay nanotubes. Langmuir 2017, 33, 13051–13059. [Google Scholar]
- Reddy, N.S.G.; Rao, K.M.; Park, S.Y.; Kim, T.; Chung, I. Fabrication of aminosilanized halloysite based floating biopolymer composites for sustained gastro retentive release of curcumin. Macromol. Res. 2019, 27, 490–496. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Paharik, A.E.; Horswill, A.R. The staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 2016, 4, 529–566. [Google Scholar] [CrossRef] [PubMed]
- Lucke, M.; Wildemann, B.; Sadoni, S.; Surke, C.; Schiller, R.; Stemberger, A.; Raschke, M.; Haas, N.P.; Schmidmaier, G. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone 2015, 36, 770–778. [Google Scholar] [CrossRef]
- McMillan, D.J.; Lutton, C.; Rosenzweig, N.; Sriprakash, K.S.; Goss, B.; Stemberger, M.; Schuetz, M.A.; Steck, R. Prevention of Staphylococcus aureus biofilm formation on metallic surgical implants via controlled release of gentamicin. J. Biomed. Sci. Eng. 2011, 4, 535–542. [Google Scholar] [CrossRef]
- Aviv, M.; Berdicevsky, I.; Zilberman, M. Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. J. Biomed. Mater. Res. A 2007, 83, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Saleh, P.; Abbasalizadeh, S.; Rezaeian, S.; Naghavi-Behzad, M.; Piri, R.; Pourfeizi, H.H. Gentamicin-mediated ototoxicity and nephrotoxicity: A clinical trial study. Niger. Med. J. 2016, 57, 347–352. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
- Buffa, S.; Bonini, M.; Ridi, F.; Severi, M.; Losi, P.; Volpi, S.; Al Kayal, T.; Soldani, G.; Baglioni, P. Design and characterization of a composite material based on Sr(II)-loaded clay nanotubes included within a biopolymer matrix. J. Coll. Interface Sci. 2015, 448, 501–507. [Google Scholar] [CrossRef]
- Sarina, N.; Kurakulab, M.; Singha, K.J.; Kumar, A. Strontium and selenium doped bioceramics incorporated polyacrylamide-carboxymethylcellulose hydrogel scaffolds: Mimicking key features of bone regeneration. J. Asian Ceramic Soc. 2021, 9, 531–548. [Google Scholar] [CrossRef]
- Martín-Del-Campo, M.; Sampedro, J.G.; Flores-Cedillo, M.L.; Rosales-Ibañez, R.; Rojo, L. Bone regeneration induced by strontium folate loaded biohybrid scaffolds. Molecules 2019, 24, 1660. [Google Scholar] [CrossRef]
- Rojo, L.; Radley-Searle, S.; Fernandez-Gutierrez, M.; Rodriguez-Lorenzo, L.M.; Abradelo, C.; Deb, S.; Roman, J.S. The synthesis and characterization of strontium and calcium folates with potential osteogenic activity. Mater. Chem. B 2015, 3, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; Ray, S.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomat 2019, 34, 8589–8598. [Google Scholar]
- Shi, H.; Zeng, S.; Liu, X.; Yu, T.; Zhou, C. Effects of strontium doping on the degradation and Sr ion release behaviors of α-tricalcium phosphate bone cement. J. Am. Chem. Soc. 2017, 101, 502–508. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Wang, J.; Huang, C.; Wang, Y.; Yang, H.; Liu, W.; Wang, T.; Wang, D.; Wang, G.; et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact. Mater. 2020, 5, 334–347. [Google Scholar] [CrossRef]
- Saeid, K.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and promising applications of strontium (Sr)-containing bioactive glasses in bone tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elumalai, A.; Mills, D.K. An Eco-Friendly, Simple, and Inexpensive Method for Metal-Coating Strontium onto Halloysite Nanotubes. J. Compos. Sci. 2022, 6, 276. https://doi.org/10.3390/jcs6090276
Elumalai A, Mills DK. An Eco-Friendly, Simple, and Inexpensive Method for Metal-Coating Strontium onto Halloysite Nanotubes. Journal of Composites Science. 2022; 6(9):276. https://doi.org/10.3390/jcs6090276
Chicago/Turabian StyleElumalai, Anusha, and David K. Mills. 2022. "An Eco-Friendly, Simple, and Inexpensive Method for Metal-Coating Strontium onto Halloysite Nanotubes" Journal of Composites Science 6, no. 9: 276. https://doi.org/10.3390/jcs6090276
APA StyleElumalai, A., & Mills, D. K. (2022). An Eco-Friendly, Simple, and Inexpensive Method for Metal-Coating Strontium onto Halloysite Nanotubes. Journal of Composites Science, 6(9), 276. https://doi.org/10.3390/jcs6090276