Heavy Metal Removal from Aqueous Solutions Using Biomaterials and/or Functional Composites: Recent Advances and the Way Forward in Wastewater Treatment Using Digitalization
Abstract
:1. Introduction
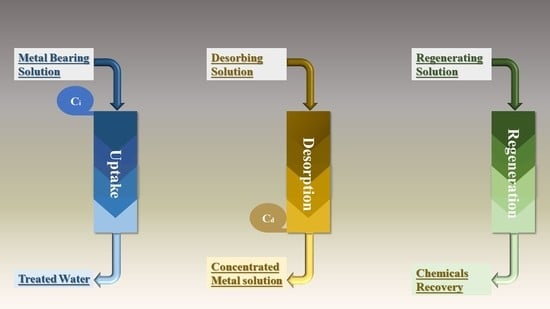
2. Heavy Metal Removal Using Biomaterials
2.1. Passive Metal-Binding Mechanisms
2.1.1. Ion Exchange
2.1.2. Surface Complexation
3. Advances in Water Treatment Using Functional Composites
3.1. Nanomaterials for Water Treatment
3.2. Technological Strengths of Nanomaterials
3.3. Types of Nanomaterials
3.3.1. nZVI
3.3.2. Dendrimer
3.3.3. Metal Organic Framework (MOF)
3.3.4. Nanoporous Materials
4. Prospect and Ways forward for Digitalization in Water Treatment Industry
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, M.; Kurniawan, T.A.; Duan, L.; Song, Y.; Hermanowicz, S.W.; Othman, M.H.D. Advances in BiOX-based ternary photocatalysts for water technology and energy storage applications: Research trends, challenges, solutions, and ways forward. Rev. Environ. Sci. Bio/Technol. 2022, 21, 331–370. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Sillanpää, M. Nanoadsorbents for remediation of aquatic environment: Local and practical solutions for global water pollution problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295. [Google Scholar] [CrossRef]
- Liang, X.; Kurniawan, T.A.; Goh, H.H.; Zhang, D.; Dai, W.; Liu, H.; Goh, K.C.; Othman, M.H.D. Conversion of landfilled waste-to-electricity (WTE) for energy efficiency improvement in Shenzhen (China): A strategy to contribute to resource recovery of unused methane for generating renewable energy on-site. J. Clean. Prod. 2022, 369, 133078. [Google Scholar] [CrossRef]
- Liang, X.; Goh, H.H.; Kurniawan, T.A.; Zhang, D.; Dai, W.; Liu, H.; Liu, J.; Goh, K.C. Utilizing landfill gas (LFG) to electrify digital data centers in China for accelerating energy transition in Industry 4.0 era. J. Clean. Prod. 2022, 369, 133297. [Google Scholar] [CrossRef]
- Sniatala, B.; Kurniawan, T.A.; Sobotka, D.; Makinia, J.; Othman, M.H.D. Macro-nutrients recovery from liquid waste as a sustainable resource for production of recovered mineral fertilizer: Uncovering alternative options to sustain global food security cost-effectively. Sci. Total Environ. 2023, 856, 159283. [Google Scholar] [CrossRef]
- Maiurova, A.; Kurniawan, T.A.; Kustikova, M.; Bykovskaia, E.; Othman, M.H.D.; Singh, D.; Goh, H.H. Promoting digital transformation in waste collection service and waste recycling in Moscow (Russia): Applying a circular economy paradigm to mitigate climate change impacts on the environment. J. Clean. Prod. 2022, 354, 131604. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Sillanpää, M. Treatment of contaminated water laden with 4-chlorophenol using coconut shell waste-based activated carbon modified with chemical agents. Sep. Sci. Technol. 2011, 46, 460–472. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Liang, X.; O’Callaghan, E.; Goh, H.; Othman, M.H.D.; Avtar, R.; Kusworo, T.D. Transformation of solid waste management in China: Moving towards sustainability through digitalization-based circular economy. Sustainability 2022, 14, 2374. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Avtar, R.; Singh, D.; Xue, W.; Dzarfan Othman, M.H.; Hwang, G.H.; Iswanto, I.; Albadarin, A.B.; Kern, A.O. Reforming MSWM in Sukunan (Yogjakarta, Indonesia): A case-study of applying a zero-waste approach based on circular economy paradigm. J. Clean. Prod. 2020, 284, 124775. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Europe, Water Sanitation and Health (WSH). 2008. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html (accessed on 12 January 2023).
- Hong Kong Environmental Protection Department (HK EPD). Technical Memorandum Standards for Effluents Discharged into Drainage and Sewerage Systems, Inland and Coastal Water. 2008. Available online: http://www.legislation.gov.hk/eng/home.htm (accessed on 12 January 2023).
- China Environmental Protection Agency (China EPA). Available online: http://english.mee.gov.cn/Resources/laws/ (accessed on 12 January 2023).
- Kurniawan, T.A.; Chan, G.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wateswater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Chan, G. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, 29, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Kurniawan, T.A.; Gui, H.; Li, H.; Wang, Y.; Li, Q. Role of CuxO-anchored pyrolyzed hydrochars on H2O2-activated degradation of tetracycline: Effects of pyrolysis temperature and pH. Ind. Eng. Chem. Res. 2022, 61, 8847–8857. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Singh, D.; Avtar, R.; Dzarfan Othman, M.H.; Hwang, G.H.; Albadarin, A.B.; Rezakazemi, M.; Setiadi, T.; Shirazian, S. Resource recovery from landfill leachate: An experimental investigation and perspectives. Chemosphere 2021, 274, 129986. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Liang, X.; Singh, D.; Othman, M.H.D.; Goh, H.H.; Gikas, P.; Kern, A.O.; Kusworo, T.D.; Shoqeir, J.A. Harnessing landfill gas (LFG) for electricity: A strategy to mitigate greenhouse gas emissions in Jakarta (Indonesia). J. Environ. Manag. 2022, 301, 113882. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Li, Q.; Gui, H.; Othman, M.H.D. Treatment of As(III)-contaminated water using iron-coated carbon fiber. Materials 2022, 15, 4365. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 122, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, T.A.; Othman, M.H.D.; Singh, D.; Avtar, R.; Goh, H.H.; Setiadi, T.; Lo, W.H. Technological solutions for long-term management of partially used nuclear fuel: A critical review. Ann. Nucl. Energy 2022, 166, 108736. [Google Scholar] [CrossRef]
- Volesky, B.; Holan, Z.R. Biosorption of heavy metal. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, D.; Volesky, B. Advances in the biosorption of heavy metals. Trends Biotechnol. 1998, 16, 291–300. [Google Scholar] [CrossRef]
- Premakumara, D.G.J.; Canete, A.L.M.L.; Nagaishi, M.; Kurniawan, T.A. Policy implementation of the Republic Act (RA) No. 9003 in the Philippines on MSW management: A case study of Cebu City. Waste Manag. 2014, 34, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, T.R.; Philip, L.; Iyengar, L.; Venkobachar, C. Application studies of biosorption for monazite processing industry effluents. Bioresour. Technol. 1994, 49, 179–186. [Google Scholar] [CrossRef]
- Wang, L.; Chua, H.; Wong, P.K.; Lo, W.; Yu, P.H.F.; Zhao, Y.G. An optimal magnetite immobilized Pseudomonas putida 5-x cell system for Cu2+ removal from industrial waste effluent. Water Sci. Technol. 2000, 41, 241–248. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solution—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Hwang, G.H.; Gikas, P. Unlocking digital technology in waste recycling industry in Industry 4.0 era: A transformation towards digitalization-based circular economy in Indonesia. J. Clean. Prod. 2022, 357, 131911. [Google Scholar] [CrossRef]
- Wang, L.; Chua, H.; Wong, P.K.; Lo, W.H.; Yu, P.H.F. Ni2+ removal and recovery from electroplating effluent by Pseudomonas putida 5-x cell biomass. J. Environ. Sci. Health Part A 2003, 38, 521–531. [Google Scholar] [CrossRef]
- Congeevaram, S.; Dhanarani, S.; Park, J.; Dexilin, M.; Thamaraiselvi, K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J. Hazard. Mater. 2007, 146, 270–277. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Micobial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Morales-Barrera, L.; Cristiani-Urbina, E. Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water Air Soil Pollut. 2008, 187, 327–336. [Google Scholar] [CrossRef]
- Leung, W.C.; Wong, M.F.; Chua, H.; Lo, W.; Yu, P.H.F.; Leung, C.K. Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Sci. Technol. 2000, 41, 233–240. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Figueira, M.M.; Volesky, B.; Ciminelli, V.S.T.; Roddick, F.A. Biosorption of metals in brown seaweed biomass. Water Res. 2000, 34, 196–204. [Google Scholar] [CrossRef]
- Wong, M.F.; Chua, H.; Lo, W.; Leung, C.K.; Yu, P.H.F. Removal and recovery of copper (II) ions by bacterial biosorption. Appl. Biochem. Biotechnol. 2001, 91, 447–457. [Google Scholar] [CrossRef]
- Goyal, N.; Jain, S.C.; Banerjee, U.C. Comparative studies on the microbial adsorption of heavy metals. Adv. Environ. Res. 2003, 7, 311–319. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Guo, S. Mechanisms of lead biosorption on cellulose/chitin beads. Water Res. 2005, 30, 3755–3762. [Google Scholar] [CrossRef]
- Lo, H.M.; Liu, M.; Pai, T.; Liu, W.; Wang, S.; Banks, C.; Hung, C.; Chiang, C.; Lin, K.; Chiu, H.; et al. Biostabilization assessment of MSW co-disposed with MSWI fly ash in anaerobic bioreactors. J. Hazard. Mater. 2009, 162, 1233–1242. [Google Scholar] [CrossRef]
- Yin, H.; He, B.; Peng, H.; Ye, J.; Yang, F.; Zhang, N. Removal of Cr(VI) and Ni(II) from aqueous solution by fused yeast: Study of cations release and biosorption mechanism. J. Hazard. Mater. 2008, 158, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xiao, D. Chemical modification and XPS study for lead(II) binding by wheat stems biomass. Sep. Sci. Technol. 2008, 43, 2196–2207. [Google Scholar] [CrossRef]
- Schneider, I.A.H.; Rubio, J.; Smith, R.W. Biosorption of metals onto plant biomass: Exchange adsorption or surface precipitation? Int. J. Miner. Proc. 2001, 62, 111–120. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Toro, L.; Vegliò, F. Olive mill solid residues as heavy metal sorbent material: A preliminary study. Waste Manag. 2002, 22, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.H.; Chua, H.; Lam, K.H.; Bi, S.P. A comparative investigation on the biosorption of lead by filamentous fungal biomass. Chemosphere 1999, 39, 2723–2736. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T. Mechanism of biosorption of heavy metals by Mucor rouxii. Eng. Life Sci. 2008, 8, 363–371. [Google Scholar] [CrossRef]
- Vasudevan, P.; Padmavathy, V.; Dhingra, S.C. Biosorption of monovalent and divalent ions on baker’s yeast. Bioresour. Technol. 2002, 82, 285–289. [Google Scholar] [CrossRef]
- Liu, T.; Li, H.; Li, Z.; Xiao, X.; Chen, L.; Deng, L. Removal of hexavalent chromium by fungal biomass of Mucor racemosus: Influencing factors and removal mechanism. World J. Microbiol. Biotehcnol. 2007, 23, 1685–1693. [Google Scholar] [CrossRef]
- Cho, D.H.; Kim, E.Y. Characterization of Pb2+ biosorption from aqueous solution by Rhodotorula glutinis. Bioproc. Biosyst. Eng. 2003, 25, 271–277. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Cheung, K.C.; Tam, N.F.Y.; Wong, M.H. A comparative study on metal sorption by brown seaweed. Chemosphere 2006, 65, 51–57. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Avtar, R.; Xu, P.; Othman, M.H.D. Recovering heavy metals from electroplating wastewater and their conversion into Zn2Cr-layered double hydroxide (LDH) for pyrophosphate removal from industrial wastewater. Chemosphere 2021, 271, 129861. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Othman, M.H.D.; Adam, M.R.; Goh, H.H.; Mohyudin, A.; Avtar, R.; Kusworo, T.D. Treatment of pulping whitewater using membrane filtrations. Chem. Papers 2022, 76, 5001–5010. [Google Scholar] [CrossRef]
- Ahmady-Asbchin, S.; Andrès, Y.; Gérente, C.; Cloirec, P.L. Biosorption of Cu(II) from aqueous solution by Fucus serratus: Surface characterization and sorption mechanisms. Bioresour. Technol. 2008, 99, 6150–6155. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B. Biosorbents for metal recovery. Trends Biotechnol. 1987, 5, 96–101. [Google Scholar] [CrossRef]
- Marin, J.; Ayele, J. Removal of some heavy metal cations from aqueous solutions by spruce sawdust. I. Study of the binding mechanisms through batch experiments. Environ. Technol. 2002, 23, 1157–1171. [Google Scholar] [CrossRef]
- Marinsky, J.A. Ion binding in charged polymers. Coord. Chem. Rev. 1976, 19, 125–171. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Maiurova, A.; Kustikova, M.; Bykovskaia, E.; Othman, M.H.D.; Goh, H.H. Accelerating sustainability transition in St. Petersburg (Russia) through digitalization-based circular economy in waste recycling industry: A strategy to promote carbon neutrality in era of Industry 4.0. J. Clean. Prod. 2022, 363, 132452. [Google Scholar] [CrossRef]
- Kuyucak, N.; Volesky, B. Accumulation of cobalt by marine alga. Biotechnol. Bioeng. 1989, 33, 809–814. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y. Mechanism of Cd2+, Cu2+ and Ni2+ biosorption by aerobic granules. Sep. Purif. Technol. 2008, 58, 400–411. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Singh, D.; Xue, W.; Avtar, R.; Othman, M.H.D.; Hwang, G.H.; Setiadi, T.; Albadarin, A.B.; Shirazian, S. Resource recovery toward sustainability through nutrient removal from landfill leachate. J. Environ. Manag. 2021, 287, 112265. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Lo, W.H.; Ng, L.M.; Chua, H.; Yu, P.H.F.; Sin, S.N.; Wong, P.K. Biosorption and desorption of copper (II) ions by Bacillus sp. Appl. Biochem. Biotechnol. 2003, 107, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Coll. Intr. Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Tunali, S.; Kiran, I.; Akar, T. Chromium(VI) biosorption characteristics of Neurospora crassa fungal biomass. Miner. Eng. 2005, 18, 681–689. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, D.H.; Park, J.M.; Volesky, B. Biosorption of trivalent chromium on the brown seaweed biomass. Environ. Sci. Technol. 2001, 35, 4353–4358. [Google Scholar] [CrossRef]
- Park, D.H.; Yun, Y.S.; Park, J.M. Reduction of hexavalent chromium with the brown seaweed Ecklonia biomass. Environ. Sci. Technol. 2004, 38, 4860–4864. [Google Scholar] [CrossRef]
- Fourest, E.; Volesky, B. Contribution of sulfonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environ. Sci. Technol. 1996, 30, 277–282. [Google Scholar] [CrossRef]
- Yuncu, B.; Sanin, F.D.; Yetis, U. An investigation of heavy metal biosorption in relation to C/N ratio of activated sludge. J. Hazard. Mater. 2006, 137, 990–997. [Google Scholar] [CrossRef]
- Teemu, H.; Seppo, S.; Jussi, M.; Raija, T.; Kalle, L. Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int. J. Food. Microbiol. 2008, 125, 170–175. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef]
- Akar, T.; Cabuk, A.; Tunali, S.; Yamac, M. Biosorption potential of the macrofungus Ganoderma carnosum for removal of lead(II) ions from aqueous solutions. J. Environ. Sci. Health Part A 2006, 41, 2587–2606. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Cano-Aquilera, I.; Webb, R.; Tiemann, K.J.; Gutiérrez-Corona, F. Copper adsorption by inactivated cells of Mucor rouxii: Effect of esterification of carboxyl group. J. Hazard. Mater. 1996, 48, 171–180. [Google Scholar] [CrossRef]
- Francesca, P.; Sara, M.; Luigi, T. New biosorbent materials for heavy metal removal: Product development guided by active site characterization. Water Res. 2008, 42, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, M.F.; Peralta-Videa, J.R.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Removal of copper, lead, and zinc from contaminated water by saltbush biomass: Analysis of the optimum binding, stripping, and binding mechanism. Bioresour. Technol. 2008, 99, 4438–4444. [Google Scholar] [CrossRef]
- Schiewer, S.; Wong, M.C. Metal binding stoichiometry and isotherm choice in biosorption. Environ. Sci. Technol. 1999, 33, 3821–3828. [Google Scholar] [CrossRef]
- Crist, R.H.; Martin, J.R.; Guptill, P.W.; Eslinger, J.M. Interaction of metals and protons with algae. Adsorption and metal displacement by protons. Environ. Sci. Technol. 1990, 24, 337–342. [Google Scholar] [CrossRef]
- Deng, S.; Ting, Y.P. Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res. 2005, 39, 2167–2177. [Google Scholar] [CrossRef]
- Guo, M.; Weng, X.; Wang, T.; Chen, Z. Biosynthesized iron-based nanoparticles used as a heterogeneous catalyst for the removal of 2, 4-dichlorophenol. Sep. Purif. Technol. 2017, 175, 222–228. [Google Scholar] [CrossRef]
- Schulte, J.; Dutta, J. Nanotechnology in environmental protection and pollution. Sci. Technol. Adv. Mater. 2005, 6, 219–220. [Google Scholar] [CrossRef]
- Masciangioli, T.; Zhang, W.X. Environmental technologies at nanoscale. Environ. Sci. Technol. 2003, 37, 102A–108A. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K. Photocatalytic degradation of agricultural N-heterocyclic organic pollutants using immobilized nanoparticles of titania. J. Hazard. Mater. 2007, 145, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laîné, J.M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl. Catal. B Environ. 2004, 50, 259–269. [Google Scholar] [CrossRef]
- Fernández-Pacheco, R.; Arruebo, M.; Marquina, C.; Ibarra, R.; Arbiol, J.; Santamaría, J. Highly magnetic silica-coated iron nanoparticles prepared by the arc-discharge method. Nanotechnology 2006, 17, 1188. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Li, G.; Fudickar, W.; Skupin, M.; Klyszcz, A.; Draeger, C.; Lauer, M.; Fuhrhop, J.H. Rigid lipid membranes and nanometer clefts: Motifs for the creation of molecular landscapes. Angew. Chem. Int. 2002, 41, 1828–1852. [Google Scholar] [CrossRef]
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube–TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- DeMarco, M.J.; SenGupta, A.K.; Greenleaf, J.E. Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res. 2003, 37, 164–176. [Google Scholar] [CrossRef]
- Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.S.; Gavaskar, A.; et al. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ. Sci. Technol. 2005, 39, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Srinivasan, M.P. Langmuir–Blodgett film fabricated with dendrimer modified polyimide. Coll. Surfaces A Physicochem. Eng. Asp. 2005, 257, 183–190. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Everett, D.H.; Fairbridge, C.; Haynes, M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Guidelines for the characterization of porous solids. Stud. Surface Sci. Catal. 1994, 87, 1–9. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Heck, R.M. Catalytic converters: State of the art and perspectives. Catal. Today 1999, 51, 351–360. [Google Scholar] [CrossRef]
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519. [Google Scholar] [CrossRef]
- Wang, Y.P.; Pei, X.W.; He, X.Y.; Yuan, K. Synthesis of well-defined, polymer-grafted silica nanoparticles via reverse ATRP. Eur. Polym. J. 2005, 41, 1326–1332. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, A. Nanotechnology: An emerging future trend in wastewater treatment with its innovative products and processes. Nanotechnology 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Roco, M.C. Nanoscale science and engineering education activities in the United States (2001–2002). J. Nanopart. Res. 2002, 4, 271. [Google Scholar] [CrossRef]
- Kalpana, T.M.; Gopalakrishnan, S. Chapter 17: Green economic and secure libraries on cloud. In Cloud Computing and Virtualization Technologies in Libraries; IGI Global: Hershey, PA, USA, 2014; pp. 297–315. [Google Scholar] [CrossRef]
- Qadir, M.; Wichelns, D.; Raschid-Sally, L.; McCornick, P.G.; Drechsel, P.; Bahri, A.; Minhas, P.S. The challenges of wastewater irrigation in developing countries. Agric. Water Manag. 2010, 97, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.K. A case study on the appropriateness of using quick response (QR) codes in libraries and museums. Libr. Inf. Sci. Res. 2013, 35, 207–215. [Google Scholar] [CrossRef]
- Chimienti, V.; Iliano, S.; Dassisti, M.; Dini, G.; Failli, F. Guidelines for implementing augmented reality procedures in assisting assembly operations. In Precision Assembly Technologies and Systems: 5th IFIP WG 5.5 International Precision Assembly Seminar, IPAS 2010, Chamonix, France, 14–17 February 2010; Springer: Berlin/Heidelberg, Germany, 2010; pp. 174–179. [Google Scholar] [CrossRef] [Green Version]
- Arsanjani, A.; Zhang, L.J.; Ellis, M.; Allam, A.; Channabasavaiah, K. A service-oriented reference architecture. IT Prof. 2007, 9, 10–17. [Google Scholar] [CrossRef]
- Mohammad, A.S.; Pradhan, M.R. Machine learning with big data analytics for cloud security. Comp. Electr. Eng. 2021, 96, 107527. [Google Scholar] [CrossRef]
- Sun, A.Y.; Scanlon, B.R. How can Big Data and machine learning benefit environment and water management: A survey of methods, applications, and future directions. Environ. Res. Lett. 2019, 14, 073001. [Google Scholar] [CrossRef]
- Sjödin, D.; Parida, V.; Kohtamäki, M.; Wincent, J. An agile co-creation process for digital servitization: A micro-service innovation approach. J. Bus. Res. 2020, 112, 478–491. [Google Scholar] [CrossRef]
- Ulfat, W.; Mohyuddin, A.; Amjad, M.; Kurniawan, T.A.; Mujahid, B.; Nadeem, S.; Javed, M.; Amjad, A.; Ashraf, A.Q.; Othman, M.H.D.; et al. Reuse of buffing dust-laden tanning waste hybridized with polystyrene for fabrication of thermal insulation materials. Sustainability 2023, 15, 1958. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Gikas, P.; Chong, K.K.; Chew, K.W. Challenges and opportunities for biochar management to promote circular economy and carbon neutrality. J. Environ. Manag. 2023, 332, 117429. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Ayub, M.; Goh, H.H.; Kusworo, T.D.K.; Mohyuddin, A.; Chew, K.W. Microbial fuel cells (MFC): A potential game changer in renewable energy development. Sustainability 2023, 14, 16847. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Goh, H.H.; Chew, K.W. From liquid waste to mineral fertilizer: Recovery, recycle and reuse of high-value macro-nutrients from landfill leachate to contribute to circular economy, food security, and carbon neutrality. Process Saf. Environ. Prot. 2023, 170, 791–807. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Meidiana, C.; Othman, M.H.D.; Goh, H.H.; Chew, K.W. Strengthening waste recycling industry in Malang (Indonesia): Lessons from waste management in the era of Industry 4.0. J. Clean. Prod. 2023, 382, 135296. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zhao, T.; Wang, X.; Isaeva, V.I.; Kustov, L.M.; Yao, J.; Gao, J. Bimetal-organic framework-derived nanotube@cellulose aerogels for peroxymonosulfate (PMS) activation. Carbohydr. Polym. 2022, 296, 119969. [Google Scholar] [CrossRef]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef]



| Heavy Metals | Potential Health Effects | MCLs (mg/L) | ||
|---|---|---|---|---|
| WHO [12] | HK EPD [13] | China’s EPA [14] | ||
| Cr(VI) | Kidney, liver damage | 0.05 | 0.05–0.1 | 0.05–0.01 |
| Cr(III) | Skin rashes | |||
| Hg(II) | Teratogenesis, liver, neural damage | 0.006 | 0.001 | 0.001 |
| Cd(II) | Anemia, emphysema, heart disease | 0.003 | 0.001 | 0.005–0.01 |
| Cu(II) | Diarrhea, kidney, liver damage | 2 | 0.05–0.1 | 1 |
| Pb(II) | Anemia, constipation, hypertension | 0.01 | 0.1 | 0.05–0.1 |
| Ni(II) | Cancer, diarrhea, insomnia | 0.07 | 0.1–0.2 | 0.05–0.1 |
| Metal Ions | Biosorbents | Functional Groups | References |
|---|---|---|---|
| Cr(II) | Ecklonia sp. | –COO− | [68] |
| Ecklonia sp. | –COO− | [69] | |
| Cd(II) | Sargassum fluitans | –COO− | [70] |
| Padina sp. and Sargassum sp. | –COO− | [66] | |
| Activated sludge | –COO− | [71] | |
| Cd(II) & Ni(II) | Aerobic granules | –COO− | [62] |
| Cd(II) & Pb(II) | Lactobacillus fermentum and Bifidobacterium longum | –COO− | [72] |
| –PO42− | |||
| Pb(II) | Pinus sylvestris | –COO− | [73] |
| Ganoderma carnosum | –PO42− | [74] | |
| Cu(II) | Mucor rouxii | –COO− | [75] |
| Padina sp. and Sargassum sp. | R–O–R | [66] | |
| Activated sludge | –R3N | [71] | |
| Olive pomace | –COO− | [76] | |
| Olive pomace (thermally treated) |  | [76] | |
| Cu(II) & Zn(II) | Atriplex canescens | –COO− | [77] |
| Zn(II) | Padina sp. and Sargassum sp. | –COO− | [66] |
| Activated sludge | –COO− –R3N | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, T.A.; Lo, W.-H.; Liang, X.; Goh, H.H.; Othman, M.H.D.; Chong, K.-K.; Mohyuddin, A.; Kern, A.O.; Chew, K.W. Heavy Metal Removal from Aqueous Solutions Using Biomaterials and/or Functional Composites: Recent Advances and the Way Forward in Wastewater Treatment Using Digitalization. J. Compos. Sci. 2023, 7, 84. https://doi.org/10.3390/jcs7020084
Kurniawan TA, Lo W-H, Liang X, Goh HH, Othman MHD, Chong K-K, Mohyuddin A, Kern AO, Chew KW. Heavy Metal Removal from Aqueous Solutions Using Biomaterials and/or Functional Composites: Recent Advances and the Way Forward in Wastewater Treatment Using Digitalization. Journal of Composites Science. 2023; 7(2):84. https://doi.org/10.3390/jcs7020084
Chicago/Turabian StyleKurniawan, Tonni Agustiono, Wai-Hung Lo, Xue Liang, Hui Hwang Goh, Mohd Hafiz Dzarfan Othman, Kok-Keong Chong, Ayesha Mohyuddin, Axel Olaf Kern, and Kit Wayne Chew. 2023. "Heavy Metal Removal from Aqueous Solutions Using Biomaterials and/or Functional Composites: Recent Advances and the Way Forward in Wastewater Treatment Using Digitalization" Journal of Composites Science 7, no. 2: 84. https://doi.org/10.3390/jcs7020084
APA StyleKurniawan, T. A., Lo, W. -H., Liang, X., Goh, H. H., Othman, M. H. D., Chong, K. -K., Mohyuddin, A., Kern, A. O., & Chew, K. W. (2023). Heavy Metal Removal from Aqueous Solutions Using Biomaterials and/or Functional Composites: Recent Advances and the Way Forward in Wastewater Treatment Using Digitalization. Journal of Composites Science, 7(2), 84. https://doi.org/10.3390/jcs7020084