Preparation and Properties of Flame-Retardant Polyurethane Pressure Sensitive Adhesive and Its Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Preparation of I-FRPU-PSA
2.4. Preparation of I-FRPU-PSA Tapes
3. Results and Discussion
3.1. FTIR Spectra
3.2. Thermal Performance Analysis
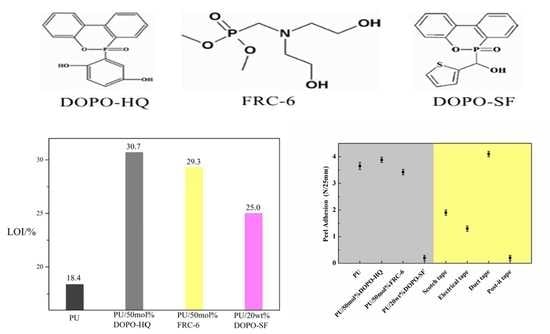
3.3. LOI and Vertical Combustion
3.4. Infrared Analysis of Residual Carbon
3.5. Adhesive Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, X.H. Progress on rubber-based pressure-sensitive adhesives. J. Adhesion. 2018, 94, 77–96. [Google Scholar] [CrossRef]
- Junhao, Z.; Xuntian, Z.; Quanzhi, M. Performance Study on Acrylic Pressure-Sensitive Adhesive Modified with Metakaolin. IOP Conf. Ser. Mater. Sci. Eng. 2020, 711, 12053–12057. [Google Scholar] [CrossRef]
- Kajtna, J.; Krajnc, M. Solvent less UV crosslinkable acrylic pressure sensitive adhesives. Int. J. Adhes. Adhes. 2011, 31, 822–831. [Google Scholar] [CrossRef]
- Jamaluddin, J.; Lee, M.C. Properties of UV-curable solvent-free pressure sensitive adhesive. J. Adhes. Sci. Technol. 2013, 27, 905–911. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K.; Piatek-Hnat, M.; Czech, Z.; Bartkowiak, M. Silicone pressure-sensitive adhesives with increased thermal resistance. J. Therm. Anal. Calorim. 2022, 147, 7719–7727. [Google Scholar] [CrossRef]
- Mapari, S.; Mestry, S.; Mhaske, S.T. Developments in pressure-sensitive adhesives: A review. Polym. Bull. 2021, 78, 4075–4108. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K.; Czech, Z.; Piatek-Hnat, M. Influence of Montmorillonite on the Properties of Silicone Pressure-Sensitive Adhesives: Preparation of a Double-Sided Tape Based on the Best Composition. Silicon 2020, 12, 1887–1893. [Google Scholar] [CrossRef]
- Wenqin, D.; Ren, W. Study on environmental-friendly waterborne polyurethane flame-retardant coating polyester fabric. Adv. Mater. Res. 2015, 1088, 455–459. [Google Scholar] [CrossRef]
- Feng, J.J.; Ge, Z.; Chai, C.P.; Wang, S.P.; Yu, D.H.; Wu, G.; Luo, Y.J. Flame retardant modification of waterborne polyurethane fabric coating agent with high hydrostatic pressure resistance. Prog. Org. Coat. 2016, 97, 91–98. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martin-Martinez, J.M. Thermoplastic polyurethane coatings made with mixtures of polyethers of different molecular weights with pressure sensitive adhesion property. Prog. Org. Coat. 2018, 118, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Fuensanta, M.; Martin-Martinez, J.M. Thermoplastic polyurethane pressure sensitive adhesives made with mixtures of polypropylene glycols of different molecular weights. Int. J. Adhes. Adhes. 2019, 88, 81–90. [Google Scholar] [CrossRef]
- Ameen, D.; Michniak-Kohn, B. Development and in vitro evaluation of pressure sensitive adhesive patch for the transdermal delivery of galantamine: Effect of penetration enhancers and crystallization inhibition. Eur. J. Pharm. Biopharm. 2019, 139, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Tirri, T.; Wilen, C.E. Flame retardant polyurethane nanocomposite: Study of clay dispersion and its synergistic effect with dolomite. J. Appl. Polym. Sci. 2013, 129, 1678–1685. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. I Met Finish 2018, 96, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Mehra, D.S.; Niyogi, U.K.; Sabharwal, S.; Singh, G. Breathability studies of electron beam curable polyurethane pressure sensitive adhesive for bio-medical application. Radiat. Phys. Chem. 2014, 103, 75–83. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, L.; Wu, J.N.; Fu, T.; Wang, Y.Z. Flame-Retardant Pressure-Sensitive Adhesives Derived from Epoxidized Soybean Oil and Phosphorus-Containing Dicarboxylic Acids. Acs Sustain. Chem. Eng. 2017, 5, 3353–3361. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Marosz, V.; Samuels, N.T.; Suib, S.L.; Sun, L.Y.; Liao, L.B. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Antosik, A.K.; Makuch, E.; Gziut, K. Influence of modified attapulgite on silicone pressure-sensitive adhesives properties. J. Polym. Res. 2022, 29. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.S.; Wang, M.X.; Liu, P.; Pan, Y.H.; Liu, D.F. Synergistic effect of an aromatic boronic acid derivative and magnesium hydroxide on the flame retardancy of epoxy resin. Polym. Degrad Stabil. 2016, 130, 257–263. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.S.; Wang, M.X.; Liu, P.; Pan, Y.H.; Liu, D.F. Synthesis of a boron/nitrogen-containing compound based on triazine and boronic acid and its flame retardant effect on epoxy resin. High Perform. Polym. 2017, 29, 513–523. [Google Scholar] [CrossRef]
- Chen, S.S.; Ai, L.H.; Zhang, T.; Liu, P.; Liu, W.S.; Pan, Y.H.; Liu, D.F. Synthesis and application of a triazine derivative containing boron as flame retardant in epoxy resins. Arab J. Chem. 2020, 13, 2982–2994. [Google Scholar] [CrossRef]
- Ai, L.H.; Chen, S.S.; Zeng, J.M.; Liu, P.; Liu, W.S.; Pan, Y.H.; Liu, D.F. Synthesis and flame retardant properties of cyclophosphazene derivatives containing boron. Polym. Degrad. Stabil. 2018, 155, 250–261. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.X.; Hu, Y.F. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stabil. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Unlu, S.M.; Tayfun, U.; Yildirim, B.; Dogan, M. Effect of boron compounds on fire protection properties of epoxy based intumescent coating. Fire Mater. 2017, 41, 17–28. [Google Scholar] [CrossRef]
- Gao, H.L.; Hu, S.; Han, H.C.; Zhang, J. Effect of Different Metallic Hydroxides on Flame-Retardant Properties of Low Density Polyethylene/Melamine Polyphosphate/Starch Composites. J. Appl. Polym. Sci. 2011, 122, 3263–3269. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q.H.; Qu, B.J. Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites. Polym. Degrad Stabil. 2009, 94, 751–756. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Song, L.; Wu, J.; Fang, S.L. Influence of Fe-MMT on the fire retarding behavior and mechanical property of (ethylene-vinyl acetate copolymer/magnesium hydroxide) composite. Polym. Adv. Technol. 2008, 19, 960–966. [Google Scholar] [CrossRef]
- Ai, L.H.; Chen, S.S.; Zeng, J.M.; Yang, L.; Liu, P. Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide. ACS OMEGA 2019, 4, 3314–3321. [Google Scholar] [CrossRef]
- Ai, L.H.; Yang, L.; Hu, J.F.; Chen, S.S.; Zeng, J.M.; Liu, P. Synergistic Flame Retardant Effect of Organic Phosphorus-Nitrogen and Inorganic Boron Flame Retardant on Polyethylene. Polym. Eng. Sci. 2020, 60, 414–422. [Google Scholar] [CrossRef]
- Ai, L.H.; Liu, J.B.; Chen, S.S.; Xu, Z.P.; Liu, P. Synthesis of melamine phenyl hypophosphite and its synergistic flame retardance with SiO2 on polypropylene. J. Therm. Anal. Calorim. 2022, 147, 6207–6217. [Google Scholar] [CrossRef]
- Liu, J.B.; Zeng, L.J.; Ai, L.H.; Feng, T.C.; Liu, P.; Xiong, Y.Q. Preparation of melamine borate coated red phosphorus microcapsules and use of zinc borate as synergistic flame retardant in polyethylene. J. Vinyl. Addit. Technol. 2022, 28, 591–603. [Google Scholar] [CrossRef]
- Lu, S.K.; Liu, J.B.; Zeng, L.J.; Ai, L.H.; Liu, P. Preparation and Characterization of Cyclodextrin Coated Red Phosphorus Double-Shell Microcapsules and Its Application in Flame Retardant Polyamide6. Polymers 2022, 14, 4101. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Ignatenko, V.Y.; Antonov, S.V.; Ilyin, S.O. Effect of surface contamination on the durability and strength of stainless steel-polyisobutylene pressure-sensitive adhesive bonds. Int. J. Adhes Adhes 2019, 95, 102434. [Google Scholar] [CrossRef]
- Mao, Z.B.; Yoshida, K.; Kim, J.W. Fast packaging by a partially-crosslinked SU-8 adhesive tape for microfluidic sensors and actuators. Sen. Actuators-Phys. 2019, 289, 77–86. [Google Scholar] [CrossRef]
- McKenna, S.T.; Hull, T.R. The fire toxicity of polyurethane foams. Fire Sci. Rev. 2016, 5, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame Retardancy of Bio-Based Polyurethanes: Opportunities and Challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef]
- Yun, G.W.; Lee, J.H.; Kim, S.H. Flame retardant and mechanical properties of expandable graphite/polyurethane foam composites containing iron phosphonate dopamine-coated cellulose. Polym Compos. 2020, 41, 2816–2828. [Google Scholar] [CrossRef]
- Feng, X.M.; Li, G.Q. UV curable, flame retardant, and pressure-sensitive adhesives with two-way shape memory effect. Polymer 2022, 249, 124835. [Google Scholar] [CrossRef]
- Gadgeel, A.A.; Mhaske, S.T. Incorporation of flame retardancy in bio-resourced mannitol based curing agent for clear pressure-sensitive adhesive. Polym. Adv. Technol. 2020, 31, 3211–3227. [Google Scholar] [CrossRef]





| Scheme 6 | Mole Ratio a | PU-P b (g) | TMP (g) | DOPO-HQ (g) | FRC-6 (g) |
|---|---|---|---|---|---|
| PU | - | 43 | 5.36 | - | - |
| PU/10mol%DOPO-HQ | 9:1:0 | 43 | 4.80 | 1.93 | - |
| PU/30mol%DOPO-HQ | 7:3:0 | 43 | 3.73 | 5.80 | - |
| PU/50mol%DOPO-HQ | 5:5:0 | 43 | 2.68 | 9.68 | - |
| PU/10mol%FRC-6 | 9:0:1 | 43 | 4.80 | - | 1.52 |
| PU/30mol%FRC-6 | 7:0:3 | 43 | 3.73 | - | 4.57 |
| PU/50mol%FRC-6 | 5:0:5 | 43 | 2.68 | - | 7.65 |
| PU/40mol%DOPO-HQ/10mol%FRC-6 | 5:4:1 | 43 | 2.68 | 7.73 | 1.52 |
| PU/25mol%DOPO-HQ/25mol%FRC-6 | 2:1:1 | 43 | 2.68 | 4.83 | 3.80 |
| PU/10mol%DOPO-HQ/40mol%FRC-6 | 5:1:4 | 43 | 2.68 | 1.93 | 6.08 |
| Samples | PU-P (g) | TMP (g) | DOPO-SF (g) |
|---|---|---|---|
| PU/5wt%DOPO-SF | 43 | 5.36 | 2.42 |
| PU/10wt%DOPO-SF | 43 | 5.36 | 4.84 |
| PU/15wt%DOPO-SF | 43 | 5.36 | 7.25 |
| PU/20wt%DOPO-SF | 43 | 5.36 | 9.67 |
| PU/25wt%DOPO-SF | 43 | 5.36 | 12.09 |
| PU/30wt%DOPO-SF | 43 | 5.36 | 14.51 |
| Samples | Tonset a (°C) | Tmax1 b (°C) | Tmax2 (°C) | Tmax3 (°C) | W800 c (%) |
|---|---|---|---|---|---|
| PU | 284.5 | 340.5 | 382.4 | - | 1.49 |
| PU/50mol%DOPO-HQ | 274.6 | 320.0 | 386.3 | - | 4.54 |
| PU/50mol%FRC-6 | 239.8 | 277.3 | 373.4 | - | 10.57 |
| PU/25mol%DOPO-HQ/25mol%FRC-6 | 252.7 | 288.3 | 363.2 | - | 7.52 |
| PU/40mol%DOPO-HQ/10mol%FRC-6 | 262.3 | 300.1 | 385.9 | - | 4.56 |
| PU/20wt%DOPO-SF | 187.6 | 190.6 | 314.0 | 379.0 | 2.14 |
| Samples | UL 94 | LOI (%) |
|---|---|---|
| PU | NR | 18.4 |
| PU/10mol%DOPO-HQ | V-2 | 23.0 |
| PU/30mol%DOPO-HQ | V-1 | 26.2 |
| PU/50mol%DOPO-HQ | V-1 | 30.7 |
| PU/10mol%FRC-6 | V-2 | 23.0 |
| PU/30mol%FRC-6 | V-1 | 26.6 |
| PU/50mol%FRC-6 | V-1 | 29.3 |
| PU/40mol%DOPO-HQ/10mol%FRC-6 | V-1 | 28.8 |
| PU/25mol%DOPO-HQ/25mol%FRC-6 | V-1 | 28.3 |
| PU/10mol%DOPO-HQ/40mol%FRC-6 | V-1 | 28.0 |
| Samples | UL 94 | LOI (%) |
|---|---|---|
| PU | NR | 18.4 |
| PU/5 wt%DOPO-SF | V-2 | 22.4 |
| PU/10 wt%DOPO-SF | V-2 | 23.8 |
| PU/15 wt%DOPO-SF | V-1 | 24.2 |
| PU/20wt%DOPO-SF | V-1 | 25.0 |
| PU/25 wt%DOPO-SF | V-1 | 24.7 |
| PU/30 wt%DOPO-SF | V-1 | 24.3 |
| Samples | Initial Viscosity (Steel Ball Number) | 180° Peel Strength (N/25 mm) |
|---|---|---|
| PU tape | 7 | 3.65 ± 0.13 |
| PU/50mol%DOPO-HQ tape | 8 | 3.88 ± 0.10 |
| PU/50mol%FRC-6 tape | 10 | 3.42 ± 0.10 |
| PU/20wt%DOPO-SF tape | – | 0.15 ± 0.10 |
| Scotch tape (commercial) | 7 | 1.90 ± 0.10 |
| Electrical tape (commercial) | 13 | 1.30 ± 0.10 |
| Duct tape (commercial) | 11 | 4.05 ± 0.10 |
| Post-it tape (commercial) | 4 | 0.20 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Yang, L.; Liu, J.; Lu, S.; Ai, L.; Dong, Y.; Ye, Z.; Liu, P. Preparation and Properties of Flame-Retardant Polyurethane Pressure Sensitive Adhesive and Its Application. J. Compos. Sci. 2023, 7, 85. https://doi.org/10.3390/jcs7020085
Zeng L, Yang L, Liu J, Lu S, Ai L, Dong Y, Ye Z, Liu P. Preparation and Properties of Flame-Retardant Polyurethane Pressure Sensitive Adhesive and Its Application. Journal of Composites Science. 2023; 7(2):85. https://doi.org/10.3390/jcs7020085
Chicago/Turabian StyleZeng, Lijuan, Liu Yang, Junbang Liu, Shangkai Lu, Lianghui Ai, Yang Dong, Zhibin Ye, and Ping Liu. 2023. "Preparation and Properties of Flame-Retardant Polyurethane Pressure Sensitive Adhesive and Its Application" Journal of Composites Science 7, no. 2: 85. https://doi.org/10.3390/jcs7020085
APA StyleZeng, L., Yang, L., Liu, J., Lu, S., Ai, L., Dong, Y., Ye, Z., & Liu, P. (2023). Preparation and Properties of Flame-Retardant Polyurethane Pressure Sensitive Adhesive and Its Application. Journal of Composites Science, 7(2), 85. https://doi.org/10.3390/jcs7020085