Engineering Ligament Scaffolds Based on PLA/Graphite Nanoplatelet Composites by 3D Printing or Braiding
Abstract
:1. Introduction
2. Materials and Methods
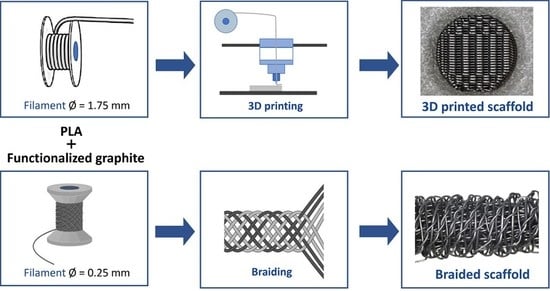
2.1. Scaffolds Production
2.1.1. Materials
2.1.2. Three-Dimensional Printing
2.1.3. Braiding
2.2. Scaffold Characterization
3. Results and Discussion
3.1. Scaffold Architecture and Morphology
3.2. Morphology of the Nanoparticle Dispersion after 3D-Printing
3.3. Scaffold Dynamic Mechanical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altman, G.H.; Horan, R.L.; Lu, H.H.; Moreau, J.; Martin, I.; Richmond, J.C.; Kaplan, D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 2002, 23, 4131–4141. [Google Scholar] [CrossRef]
- Kuo, C.K.; Marturano, J.E.; Tuan, R.S. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sport. Med. Arthros. Rehab. Ther. Technol. 2010, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrigno, B.; Bordett, R.; Duraisamy, N.; Moskow, J.; Arul, M.R.; Rudraiah, S.; Nukavarapu, S.P.; Vella, A.T.; Kumbar, S.G. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration. Bioact. Mater. 2020, 5, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Yang, F.; Goh, J.C.; Ramakrishna, S.; Lee, E.H. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed Mater. Res. A 2006, 77, 639–652. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Freeman, J.W. Ligament tissue engineering: An evolutionary materials science approach. Biomaterials 2005, 26, 7530–7536. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef]
- Freeman, J.W.; Woods, M.D.; Laurencin, C.T. Tissue engineering of the anterior cruciate ligament using a braid-twist scaffold design. J. Biomech. 2007, 40, 2029–2036. [Google Scholar] [CrossRef] [Green Version]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pinto, A.M.; Machado, A.V.; Moreira, J.A.; Gonçalves, I.C.; Magalhães, F.D. Biocompatible reinforcement of poly(Lactic acid) with graphene nanoplatelets. Polym. Compos. 2018, 39, E308–E320. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.T. Graphene-based nano composites and their applications. A review. Biosens. Bioelectron. 2019, 141, 111384. [Google Scholar] [CrossRef]
- Kim, I.-H.; Jeong, Y.G. Polylactide/exfoliated graphite nanocomposites with enhanced thermal stability, mechanical modulus, and electrical conductivity. J. Polym. Sci. B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Paiva, M.C.; Simon, F.; Novais, R.M.; Ferreira, T.; Proença, M.F.; Xu, W.; Besenbacher, F. Controlled Functionalization of Carbon Nanotubes by a Solvent-free Multicomponent Approach. ACS Nano 2010, 4, 7379–7386. [Google Scholar] [CrossRef]
- Novais, R.M.; Simon, F.; Pötschke, P.; Villmow, T.; Covas, J.A.; Paiva, M.C. Poly(lactic acid) Composites with Poly(lactic acid)-Modified Carbon Nanotubes. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3740–3750. [Google Scholar] [CrossRef]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Caetano, G.F.; Wang, W.; Chiang, W.-H.; Cooper, G.; Diver, C.; Blaker, J.J.; Frade, M.A.C.; Bártolo, P. 3D-Printed Poly(ɛ-caprolactone)/Graphene Scaffolds Activated with P1-Latex Protein for Bone Regeneration. 3D Print. Addit. Manuf. 2018, 5, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Kwiatkowski, R.; Piekarczyk, W.; Hajduga, M.B.; Kopeć, J.; Sidzina, M.; Menaszek, E. Scaffolds modified with graphene as future implants for nasal cartilage. J. Mater. Sci. 2020, 55, 4030–4042. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Parchi, P.D.; Vittorio, O.; Andreani, L.; Battistini, P.; Piolanti, N.; Marchetti, S.; Poggetti, A.; Lisanti, M. Nanoparticles for Tendon Healing and Regeneration: Literature Review. Front. Aging Neurosci. 2016, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Buckthorpe, M.; La Rosa, G.; Villa, F.D. Restoring knee extensor strength after anterior cruciate ligament reconstruction: A clinical commentary. Int. J. Sport. Phys. Ther. 2019, 14, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Imoto, A.M.; Peccin, S.; Almeida, G.J.; Saconato, H.; Atallah, Á.N. Effectiveness of electrical stimulation on rehabilitation after ligament and meniscal injuries: A systematic review. Sao Paulo Med. J. 2011, 129, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadegari-Dehkordi, S.; Sadeghi, H.R.; Attaran-Kakhki, N.; Shokouhi, M.; Sazgarnia, A. Silver nanoparticles increase cytotoxicity induced by intermediate frequency low voltages. Electromagn. Biol. Med. 2015, 34, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Gomes, C.; Pinho, I.; Gonçalves, H.; Vale, A.C.; Covas, J.A.; Alves, N.M.; Paiva, M.C. Poly(Lactic Acid)/Graphite Nanoplatelet Nanocomposite Filaments for Ligament Scaffolds. Nanomaterials 2021, 11, 2796. [Google Scholar] [CrossRef]
- Silva, M.; Ribeiro, D.; Cunha, E.; Proença, M.F.; Young, R.J.; Paiva, M.C. A Simple Method for Anchoring Silver and Copper Nanoparticles on Single Wall Carbon Nanotubes. Nanomaterials 2019, 9, 1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwadamilola, A.; Yousaf, S.; Zare, M.; Mozafari, M.; Youseffi, M.; Twigg, P.; Sefat, F. Scaffolds for ligament tissue engineering. In Handbook of Tissue Engineering Scaffolds, 1st ed.; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing Series in Biomaterials: Amsterdam, The Netherlands, 2019; Volume 1, pp. 299–327. [Google Scholar]
- Santos, M.L.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Biomaterials as Tendon and Ligament Substitutes: Current Development. In Regenerative Strategies for the Treatment of Knee Joint Disabilities, 1st ed.; Oliveira, J.M., Reis, R.L., Eds.; Springer: Cham, Switzerland, 2017; Volume 21, pp. 349–371. [Google Scholar]
- Liu, H.; Fan, H.; Wang, Y.; Toh, S.L.; Goh, J.C. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials 2008, 29, 662–674. [Google Scholar] [CrossRef]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Textile Technologies and Tissue Engineering: A Path Toward Organ Weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.J.; Im, H.; Kim, S.H.; Park, J.W.; Jung, Y. Toward Biomimetic Scaffolds for Tissue Engineering: 3D Printing Techniques in Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 586406. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Ghilan, A.; Chiriac, A.P.; Nita, L.E.; Rusu, A.G.; Neamtu, I.; Chiriac, V.M. Trends in 3D Printing Processes for Biomedical Field: Opportunities and Challenges. J. Polym. Environ. 2020, 28, 1345–1367. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. A Digital 3D Microstructural Voyage into Scaffolds: A Systematic Review of the Reported Methods and Results. Biomater. Res. 2018, 22, 26. [Google Scholar] [CrossRef]
- Laurent, C.; Liu, X.; De Isla, N.; Wang, X.; Rahouadj, R. Defining a scaffold for ligament tissue engineering: What has been done, and what still needs to be done. J. Cell. Immunother. 2018, 4, 4–9. [Google Scholar] [CrossRef]
- Mengsteab, P.Y.; Nair, L.S.; Laurencin, C.T. The past, present and future of ligament regenerative engineering. Regen. Med. 2016, 11, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Ge, Z.; Goh, J.C.; Wang, L.; Tan, E.P.; Lee, E.H. Characterization of knitted polymeric scaffolds for potential use in ligament tissue engineering. J. Biomater. Sci. Polym. Ed. 2005, 16, 1179–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Qi, Y.Y.; Wang, L.L.; Yin, Z.; Yin, G.L.; Zou, X.H.; Ouyang, H.W. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials 2008, 29, 3683–3692. [Google Scholar] [CrossRef]
- Khalil, W.A.; Sherif, H.H.A.; Hemdan, B.A.; Khalil, S.K.H.; Hotaby, W.E. Biocompatibility enhancement of graphene oxide-silver nanocomposite by functionalisation with polyvinylpyrrolidone. IET Nanobiotechnol. 2019, 13, 816–823. [Google Scholar] [CrossRef]
- De Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [Google Scholar] [CrossRef]
- Peixoto, T.; Paiva, M.C.; Marques, A.T.; Lopes, M.A. Potential of Graphene–Polymer Composites for Ligament and Tendon Repair: A Review. Adv. Eng. Mater. 2020, 22, 2000492. [Google Scholar] [CrossRef]
- Zhou, T.; Grimshaw, P.N.; Jones, C. A biomechanical investigation of the anteromedial and posterolateral bands of the porcine anterior cruciate ligament. Proc. Inst. Mech. Eng. H 2009, 223, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V. Biodegradable Polymer Nanocomposites Reinforced with Carbon Nanostructures, PLA/CNT-COOH and PLA/GNP, for Augmentation Ligament Devices: Production and Characterization. Ph.D. Thesis, Faculdade de Engenharia da Universidade do Porto, Porto, Portugal, 2016. [Google Scholar]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Wu, P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon 2010, 48, 3834–3839. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Bergeret, A.; Longerey, M.; Che, J. Preparation of polylactide/graphene composites from liquid-phase exfoliated graphite sheets. Polym. Compos. 2014, 35, 396–403. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Sampathkumar, T.S.; Verma, R.S. Modified graphene oxide nanoplates reinforced 3D printed multifunctional scaffold for bone tissue engineering. Biomater. Adv. 2022, 134, 112587. [Google Scholar] [CrossRef] [PubMed]
- Gunes, O.C.; Kara, A.; Baysan, G.; Husemoglu, R.B.; Akokay, P.; Albayrak, A.Z.; Ergur, B.C.; Havitcioglu, H. Fabrication of 3D Printed poly(lactic acid) strut and wet-electrospun cellulose nano fiber reinforced chitosan-collagen hydrogel composite scaffolds for meniscus tissue engineering. J. Biomater. Appl. 2022, 37, 683–697. [Google Scholar] [CrossRef]
- Almeida, L.R.; Martins, A.R.; Fernandes, E.M.; Oliveira, M.B.; Correlo, V.M.; Pashkuleva, I.; Marques, A.P.; Ribeiro, A.S.; Durães, N.F.; Silva, C.J.; et al. New biotextiles for tissue engineering: Development, characterization and in vitro cellular viability. Acta Biomater. 2013, 9, 8167–8181. [Google Scholar] [CrossRef]
- Peixoto, T.; Nunes, J.; Lopes, M.A.; Marinho, E.; Proença, M.F.; Lopes, P.E.; Paiva, M.C. Poly(lactic acid) composites with few layer graphene produced by noncovalent chemistry. Polym. Compos. 2022, 43, 8409–8425. [Google Scholar] [CrossRef]
- Saleh, M.; Anwar, S.; Al-Ahmari, A.M.; Alfaify, A. Compression Performance and Failure Analysis of 3D-Printed Carbon Fiber/PLA Composite TPMS Lattice Structures. Polymers 2022, 14, 4595. [Google Scholar] [CrossRef]
- Shearer, T.; Parnell, W.J.; Lynch, B.; Screen, H.R.C.; Abrahams, D.A. Recruitment Model of Tendon Viscoelasticity That Incorporates Fibril Creep and Explains Strain-Dependent Relaxation. ASME J. Biomech. Eng. 2020, 142, 071003. [Google Scholar] [CrossRef] [Green Version]
- Costa, U.O.; Nascimento, L.F.C.; Almeida Bezerra, W.B.; de Oliveira Aguiar, V.; Pereira, A.C.; Monteiro, S.N.; Pinheiro, W.A. Dynamic Mechanical Behavior of Graphene Oxide Functionalized Curaua Fiber-Reinforced Epoxy Composites: A Brief Report. Polymers 2021, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Najafidoust, M.; Hashemi, A.; Oskui, I.Z. Dynamic viscoelastic behavior of bovine periodontal ligament in compression. J. Periodont. Res. 2020, 55, 651–659. [Google Scholar] [CrossRef]
- Netti, P.; D’Amore, A.; Ronca, D.; Ambrosio, L.; Nicolais, L. Structure-mechanical properties relationship of natural tendons and ligaments. J. Mater. Sci. Mater. Med. 1996, 7, 525–530. [Google Scholar] [CrossRef]
- Edwards, J.H.; Ingham, E.; Herbert, A. Decellularisation affects the strain rate dependent and dynamic mechanical properties of a xenogeneic tendon intended for anterior cruciate ligament replacement. J. Mech. Behav. Biomed Mater. 2019, 91, 18–23. [Google Scholar] [CrossRef] [PubMed]












| Scaffold | Mean Porosity (%) | Mean Filament Thickness (µm) | Mean Pore Size (µm) | ||
|---|---|---|---|---|---|
| 3D-printed | PLA | 66.8 ± 1.5 | 240± 3 | 484 ± 4 | |
| PLA+0.5 | [(f-EG)+Ag] | 70.5 ± 1.7 | 229 ± 8 | 496 ± 7 | |
| PLA+2 | 68.9 ± 0.8 | 236 ± 7 | 485 ± 17 | ||
| Braided | PLA | 87.6 ± 0.7 | 264 ± 34 | 1035 ± 411 | |
| PLA+0.5 | [(f-EG)+Ag] | 83.4 ± 2.5 | 225 ± 33 | 1154 ± 8 | |
| PLA+1 | 87.8 ± 3.8 | 267 ± 33 | 1164 ± 545 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Pinho, I.; Gonçalves, H.; Vale, A.C.; Paiva, M.C.; Alves, N.M.; Covas, J.A. Engineering Ligament Scaffolds Based on PLA/Graphite Nanoplatelet Composites by 3D Printing or Braiding. J. Compos. Sci. 2023, 7, 104. https://doi.org/10.3390/jcs7030104
Silva M, Pinho I, Gonçalves H, Vale AC, Paiva MC, Alves NM, Covas JA. Engineering Ligament Scaffolds Based on PLA/Graphite Nanoplatelet Composites by 3D Printing or Braiding. Journal of Composites Science. 2023; 7(3):104. https://doi.org/10.3390/jcs7030104
Chicago/Turabian StyleSilva, Magda, Isabel Pinho, Hugo Gonçalves, Ana C. Vale, Maria C. Paiva, Natália M. Alves, and José A. Covas. 2023. "Engineering Ligament Scaffolds Based on PLA/Graphite Nanoplatelet Composites by 3D Printing or Braiding" Journal of Composites Science 7, no. 3: 104. https://doi.org/10.3390/jcs7030104
APA StyleSilva, M., Pinho, I., Gonçalves, H., Vale, A. C., Paiva, M. C., Alves, N. M., & Covas, J. A. (2023). Engineering Ligament Scaffolds Based on PLA/Graphite Nanoplatelet Composites by 3D Printing or Braiding. Journal of Composites Science, 7(3), 104. https://doi.org/10.3390/jcs7030104