The Surface Behavior of ZnO Films Prepared at Room Temperature
Abstract
:1. Introduction
2. Experiments and Film Structures
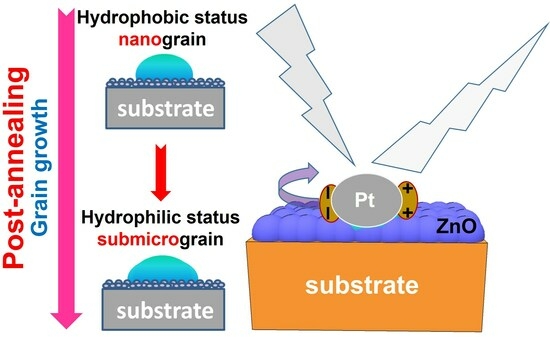
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Supin, K.K.; Namboothiri, P.M.P.; Vasundhara, M. Enhanced photocatalytic activity in ZnO nanoparticles developed using novel Lepidagathis ananthapuramensis leaf extract. RSC Adv. 2023, 13, 1497–1515. [Google Scholar]
- Zhao, F.; Lin, J.; Lei, Z.; Yi, Z.; Qin, F.; Zhang, J.; Liu, L.; Wu, X.; Yang, W.; Wu, P. Realization of 18.97% theoretical efficiency of 0.9 μm thick c-Si/ZnO heterojunction ultrathin-film solar cells via surface plasmon resonance enhancement. Phys. Chem. Chem. Phys. 2022, 24, 4871–4880. [Google Scholar] [CrossRef] [PubMed]
- Mardosaitė, R.; Jurkevičūtė, A.; Račkauskas, S. Superhydrophobic ZnO Nanowires: Wettability Mechanisms and Functional Applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Mei, G.; Menon, P.S.; Hegde, G. ZnO for performance enhancement of surface plasmon resonance biosensor: A review. Mater. Res. Express 2020, 7, 012003. [Google Scholar] [CrossRef]
- Cestellos-Blanco, S.; Zhang, H.; Kim, J.M.; Shen, Y.X.; Yang, P. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis. Nat. Catal. 2020, 3, 245–255. [Google Scholar] [CrossRef]
- Gupta, A.K.; Hsu, C.H.; Chen, C.H.; Purwidyantri, A.; Prabowo, B.A.; Wang, J.L.; Tian, Y.C.; Lai, C.S. Au-spotted zinc oxide nano-hexagonrods structure for plasmon-photoluminescence sensor. Sens. Actuator B-Chem. 2019, 290, 100–109. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Purwidyantri, A.; Liu, K.C. Surface plasmon resonance optical sensor: A review on light source technology. Biosensors 2018, 8, 80. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, T.; Palani, I.A.; Nakamura, D.; Higashihata, M.; Singh, V. Utilization of surface plasmon resonance of Au/Pt nanoparticles for highly photosensitive ZnO nanorods network based plasmon field effect transistor. Phys. E 2017, 93, 97–104. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.W.; Tian, L.; Wang, S.Q.; Gao, N.; Zhang, W.L.; Wang, P.; Yin, X.P.; Li, G.T. Facile fabrication of highly controllable gating systems based on the combination of inverse opal structure and dynamic covalent chemistry. Nanoscale 2017, 9, 7268–7275. [Google Scholar] [CrossRef]
- Lai, Y.K.; Huang, J.Y.; Cui, Z.Q.; Ge, M.Z.; Zhang, K.Q.; Chen, Z.; Chi, L.F. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef]
- Ma, Q.L.; Cheng, H.F.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.J.; Liu, Z.J.; Kang, C.F.; Lin, S.J.; He, J.H. Surface effect on resistive switching behaviors of ZnO. Appl. Phys. Lett. 2011, 99, 192106. [Google Scholar] [CrossRef]
- Chi, P.W.; Wei, D.H.; Wu, S.H.; Chen, Y.Y.; Yao, Y.D. Photoluminescence and wettability control of NiFe/ZnO heterostructure bilayer films. RSC Adv. 2015, 5, 96705–96713. [Google Scholar] [CrossRef]
- Peng, K.Y.; Ho, Y.H.; Wei, D.H.; Yu, Y.C.; Yao, Y.D.; Tian, W.C.; Wei, P.K. Efficiency enhancement of organic light-emitting devices by using honeycomb metallic electrodes and two-dimensional photonic crystal arrays. Org. Electron. 2014, 15, 3043–3051. [Google Scholar] [CrossRef]
- Lin, C.A.; Tsai, D.S.; Chen, C.Y.; He, J.H. Significant enhancement of yellow–green light emission of ZnO nanorod arrays using Ag island films. Nanoscale 2011, 3, 1195–1199. [Google Scholar] [CrossRef]
- Chen, S.C.; Wei, D.H. Controlling Surface Wettability and Plasmonic Resonance of Au/ZnO Heterostructured Films. J. Compos. Sci. 2022, 6, 328. [Google Scholar] [CrossRef]
- Wei, D.H.; Tong, S.K.; Chen, S.C.; Hao, Y.H.; Wu, M.R.; Yang, C.J.; Huang, R.T.; Chung, R.J. Tuning surface plasmonic resonance and surface wettability of Au/CrN films by nitrogen-containing gas. Nanomaterials 2022, 12, 2575. [Google Scholar] [CrossRef]
- Li, Q.; Meng, J.; Huang, J.; Li, Z. Plasmon-Induced Pyro-Phototronic Effect Enhancement in Self-Powered UV–Vis Detection with a ZnO/CuO p–n Junction Device. Adv. Funct. Mater. 2021, 32, 2108903. [Google Scholar] [CrossRef]
- Wei, D.H.; Lin, T.K.; Liang, Y.C.; Chang, H.W. Formation and Application of Core-Shell of FePt-Au Magnetic-Plasmonic Nanoparticles. Front. Chem. 2021, 9, 653718. [Google Scholar] [CrossRef]
- Pan, K.Y.; Wei, D.H. Optoelectronic and electrochemical properties of vanadium pentoxide synthesized by vapor-solid process. Nanomaterials 2016, 6, 140. [Google Scholar] [CrossRef]
- Klingshirn, C. The Luminescence of ZnO under High One- and Two-Quantum Excitation. Phys. Status Solidi B 1975, 71, 547–556. [Google Scholar] [CrossRef]
- Li, H.; Zheng, M.J.; Liu, S.D.; Ma, L.; Zhu, C.Q.; Xiong, Z.Z. Reversible surface wettability transition between superhydrophobicity and superhydrophilicity on hierarchical micro/nanostructure ZnO mesh films. Surf. Coat. Technol. 2013, 224, 88–92. [Google Scholar] [CrossRef]
- Li, J.; Jing, Z.J.; Yang, Y.X.; Zha, F.; Yan, L.; Lei, Z.Q. Reversible low adhesive to high adhesive superhydrophobicity transition on ZnO nanoparticle surfaces. Appl. Surf. Sci. 2014, 289, 1–5. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Lu, S.X.; Xu, W.G.; Cheng, Y.Y. Controllable wettability and morphology of electrodeposited surfaces on zinc substrates. Appl. Surf. Sci. 2016, 360, 904–914. [Google Scholar] [CrossRef]
- Zhao, D.F.; Jia, R.; Gao, N.K.; Yan, W.S.; Zhang, L.; Li, X.; Liu, D. Near-infrared promoted wettability recovery of superhydrophilic ZnO. J. Phys. Chem. C 2017, 121, 12745–12749. [Google Scholar] [CrossRef]
- Chen, X.Q.; Wu, Z.S.; Liu, D.D.; Gao, Z.Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.Y.; Lin, W.; Moon, K.S.; Wong, C.P. Reversible superhydrophobic–superhydrophilic transition of ZnO nanorod/epoxy composite films. ACS Appl. Mater. Interf. 2012, 4, 3959–3964. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Luo, P.; Chen, W.Z.; Pan, S.R.; Chen, D.H. Hemocompatibility of ZnO thin films prepared by filtered cathodic vacuum arc deposition. Vacuum 2013, 89, 220–224. [Google Scholar] [CrossRef]
- Myint, M.T.Z.; Kumar, N.S.; Hornyak, G.L.; Dutta, J. Hydrophobic/hydrophilic switching on zinc oxide micro-textured surface. Appl. Surf. Sci. 2013, 264, 344–348. [Google Scholar] [CrossRef]
- Xu, C.L.; Fang, L.; Wu, F.; Huang, Q.L.; Yin, B. Wetting behavior of triethoxyoctylsilane modified ZnO nanowire films. Colloid Surf. A-Physicochem. Eng. Asp. 2014, 444, 48–53. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuator B-Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Chao, C.H.; Weng, W.J.; Wei, D.H. Enhanced UV photodetector response and recovery times using a non-polar ZnO sensing layer. J. Vac. Sci. Technol. A 2016, 34, 02D106. [Google Scholar] [CrossRef]
- Chao, C.H.; Wei, D.H. Synthesis and Characterization of High c-Axis ZnO Thin Film by Plasma Enhanced Chemical Vapor Deposition System and Its UV Photodetector Application. J. Vis. Exp. 2015, 104, e53097. [Google Scholar]
- Jindal, K.; Tomar, M.; Gupta, V. Inducing electrocatalytic functionality in ZnO thin film by N doping to realize a third generation uric acid biosensor. Biosens. Bioelectron. 2014, 55, 57–65. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Zhao, D.X.; Li, B.H.; Zhang, Z.Z.; Jiang, M.M.; Shen, D.Z. High responsivity ZnO nanowires based UV detector fabricated by the dielectrophoresis method. Sens. Actuator B-Chem. 2012, 166–167, 12–16. [Google Scholar] [CrossRef]
- Chang, W.Y.; Lin, C.A.; He, J.H.; Wu, T.B. Resistive switching behaviors of ZnO nanorod layers. Appl. Phys. Lett. 2010, 96, 242109. [Google Scholar]
- Chi, P.W.; Su, C.W.; Wei, D.H. Internal Stress Induced Natural Self-Chemisorption of ZnO Nanostructured Films. Sci. Rep. 2017, 7, 43281. [Google Scholar] [CrossRef]
- Chi, P.W.; Su, C.W.; Wei, D.H. Control of Hydrophobic Surface and Wetting States in Ultra-Flat ZnO Films by GLAD Method. Appl. Surf. Sci. 2017, 404, 380–387. [Google Scholar] [CrossRef]
- Rezaie, M.N.; Manavizadeh, N.; Abadi, E.M.N.; Nadimi, E.; Boroumand, F.A. Comparison study of transparent RF-sputtered ITO/AZO and ITO/ZnO bilayers for near UV-OLED applications. Appl. Surf. Sci. 2017, 392, 549–556. [Google Scholar] [CrossRef]
- Chao, C.H.; Chi, P.W.; Wei, D.H. Investigations on the crystallographic orientation induced surface morphology evolution of ZnO thin films and their wettability and conductivity. J. Phys. Chem. C 2016, 120, 8210–8219. [Google Scholar] [CrossRef]
- Chao, C.H.; Wei, D.H. Growth of Non-Polar ZnO Thin Films with Different Working Pressures by Plasma Enhanced Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2014, 53, 11RA05. [Google Scholar] [CrossRef]
- Quan, Z.Y.; Liu, X.; Qi, Y.; Song, Z.L.; Qi, S.F.; Zhou, G.W.; Xu, X.H. Robust room temperature ferromagnetism and band gap tuning in nonmagnetic Mg doped ZnO films. Appl. Surf. Sci. 2017, 399, 751–757. [Google Scholar] [CrossRef]
- Navale, Y.H.; Navale, S.T.; Ramgir, N.S.; Stadler, F.J.; Gupta, S.K.; Aswal, D.K.; Patil, V.B. Zinc oxide hierarchical nanostructures as potential NO2 sensors. Sens. Actuator B-Chem. 2017, 251, 551–563. [Google Scholar] [CrossRef]
- Alema, F.; Ledyaev, O.; Miller, R.; Beletsky, V.; Osinsky, A.; Schoenfeld, W.V. Growth of high Mg content wurtzite MgZnO epitaxial films via pulsed metal organic chemical vapor deposition. J. Cryst. Growth 2016, 435, 6–11. [Google Scholar] [CrossRef]
- Opel, M.; Geprägs, S.; Althammer, M.; Brenninger, T.; Gross, R. Laser molecular beam epitaxy of ZnO thin films and heterostructures. J. Phys. D Appl. Phys. 2014, 47, 034002. [Google Scholar] [CrossRef]
- Flickyngerova, S.; Netrvalova, M.; Novotny, I.; Bruncko, J.; Gaspierik, P.; Sutta, P.; Tvarozek, V. Ion sputter etching of ZnO:Ga thin film surfaces. Vacuum 2012, 86, 703–706. [Google Scholar] [CrossRef]
- Montero, M.M.; Borras, A.; Saghi, Z.; Espinos, J.P.; Barranco, A.; Cotrino, J.; Elipe, A.R.G. Vertical and tilted Ag-NPs@ZnO nanorods by plasma-enhanced chemical vapor deposition. Nanotechnology 2012, 23, 255303. [Google Scholar] [CrossRef]
- Nandihalli, N. Thermoelectric films and periodic structures and spin Seebeck effect systems: Facets of performance optimization. Mater. Today Energy 2022, 25, 100965. [Google Scholar]
- Chen, X.; Zhou, Z.; Lin, Y.H.; Nan, C. Thermoelectric thin films: Promising strategies and related mechanism on boosting energy conversion performance. J. Mater. 2020, 6, 494–512. [Google Scholar] [CrossRef]
- Ghosh, R.; Basak, D.; Fujihara, S. Effect of substrate-induced strain on the structural, electrical, and optical properties of polycrystalline ZnO thin films. J. Appl. Phys. 2004, 96, 2689–2692. [Google Scholar] [CrossRef]
- Chi, P.W.; Wei, D.H. Dielectric Enhancement with Low Dielectric Loss in Textured ZnO Films Inserted with NiFe. J. Mater. Chem. C 2017, 5, 1394–1401. [Google Scholar]
- Chi, P.W.; Wei, D.H.; Yu, C.C.; Yao, Y.D. Magnetic-control-electric and reversal behavior of ZnO/NiFe/ZnO multilayer films. AIP Adv. 2017, 7, 056309. [Google Scholar] [CrossRef]
- Wang, N.W.; Yang, Y.H.; Yang, G.W. Great blue-shift of luminescence of ZnO nanoparticle array constructed from ZnO quantum dots. Nanoscale Res. Lett. 2011, 6, 338. [Google Scholar] [CrossRef] [PubMed]
- Layek, A.; De, S.; Thorat, R.; Chowdhury, A. Spectrally resolved photoluminescence imaging of ZnO nanocrystals at single-particle levels. J. Phys. Chem. Lett. 2011, 2, 1241–1247. [Google Scholar] [CrossRef]
- Makhal, A.; Sarkar, S.; Bora, T.; Baruah, S.; Dutta, J.; Raychaudhuri, A.K.; Pal, S.K. Role of resonance energy transfer in light harvesting of zinc oxide-based dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 10390–10395. [Google Scholar] [CrossRef]
- Bai, X.J.; Wang, L.; Zong, R.L.; Lv, Y.H.; Sun, Y.Q.; Zhu, Y.F. Performance enhancement of ZnO photocatalyst via synergic effect of surface oxygen defect and graphene hybridization. Langmuir 2013, 29, 3097–3105. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Oparicheva, U.G.; Grishina, A.E.; Maevskaya, M.V.; Emeline, A.V.; Bahnemann, D.W. Dependences of ZnO photoinduced hydrophilic conversion on light intensity and wavelengths. J. Phys. Chem. C 2015, 119, 9824–9828. [Google Scholar] [CrossRef]
- Swaminathan, N.; Sharma, N.; Nerthigan, Y.; Wu, H.F. Self-assembled diphenylalanine-zinc oxide hybrid nanostructures as a highly selective luminescent biosensor for trypsin detection. Appl. Surf. Sci. 2021, 554, 149600. [Google Scholar]
- Tripathy, N.; Kim, D.H. Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 2018, 5, 27. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Ibraheem, I.J.; Obaid, A.S.; Bououdina, M. Nanostructured ZnO-based biosensor: DNA immobilization and hybridization. Sens. Bio-Sens. Res. 2017, 15, 46–52. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Din, H.; Xiong, H.M. Folic acid functionalized ZnO quantum dots for targeted cancer cell imaging. Nanotechnology 2015, 26, 305702. [Google Scholar] [CrossRef]
- Choi, A.; Kim, K.; Jung, H.I.; Lee, S.Y. ZnO nanowire biosensors for detection of biomolecular interactions in enhancement mode. Sens. Actuator B-Chem. 2010, 148, 577–582. [Google Scholar] [CrossRef]
- Blažeka, D.; Radičić, R.; Maletić, D.; Živković, S.; Momčilović, M.; Krstulović, N. Enhancement of Methylene Blue Photodegradation Rate Using Laser Synthesized Ag-Doped ZnO Nanoparticles. Nanomaterials 2022, 12, 2677. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energ. Rev. 2018, 81, 536–551. [Google Scholar]
- Kadi, M.W.; Kinney, D.M.; Mohamed, R.M.; Mkhalid, I.A.; Sigmund, W. Fluorine doped zinc oxide nanowires: Enhanced photocatalysts degrade malachite green dye under visible light conditions. Ceram. Int. 2016, 42, 4672–4678. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar]
- Chen, K.H.; Pu, Y.C.; Chang, K.D.; Liang, Y.F.; Liu, C.M.; Yeh, J.W.; Shih, H.C.; Hsu, Y.J. Ag-Nanoparticle-Decorated SiO2 Nanospheres Exhibiting Remarkable Plasmon-Mediated Photocatalytic Properties. J. Phys. Chem. C 2012, 116, 19039. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar]
- Giannini, V.; Fernnández-Domínguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic Nanoantennas: Fundamentals and Their Use in Controlling the Radiative Properties of Nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar]
- Campion, A.; Kambhampati, P. Surface-Enhanced Raman Scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Scott, J.F. UV resonant Raman scattering in ZnO. Phys. Rev. B 1970, 2, 1209–1211. [Google Scholar] [CrossRef]
- Alim, K.A.; Fonoberov, V.A.; Balandin, A.A. Origin of the optical phonon frequency shifts in ZnO quantum dots. Appl. Phys. Lett. 2005, 86, 053103. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.-H.; Tong, S.-K.; Chen, S.-C.; Huang, R.-T. The Surface Behavior of ZnO Films Prepared at Room Temperature. J. Compos. Sci. 2023, 7, 335. https://doi.org/10.3390/jcs7080335
Wei D-H, Tong S-K, Chen S-C, Huang R-T. The Surface Behavior of ZnO Films Prepared at Room Temperature. Journal of Composites Science. 2023; 7(8):335. https://doi.org/10.3390/jcs7080335
Chicago/Turabian StyleWei, Da-Hua, Sheng-Kai Tong, Sheng-Chiang Chen, and Rong-Tan Huang. 2023. "The Surface Behavior of ZnO Films Prepared at Room Temperature" Journal of Composites Science 7, no. 8: 335. https://doi.org/10.3390/jcs7080335
APA StyleWei, D. -H., Tong, S. -K., Chen, S. -C., & Huang, R. -T. (2023). The Surface Behavior of ZnO Films Prepared at Room Temperature. Journal of Composites Science, 7(8), 335. https://doi.org/10.3390/jcs7080335