Bio-Oil-Based Epoxy Resins from Thermochemical Processing of Sustainable Resources: A Short Review
Abstract
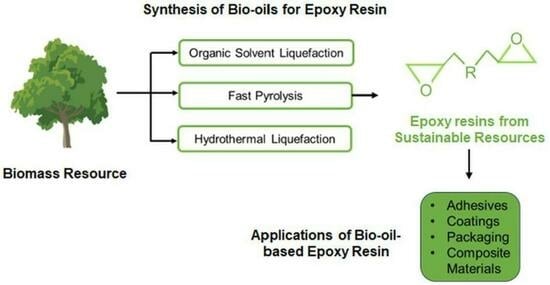
:1. Introduction
2. Thermochemical Approaches to Produce Bio-Oils for Epoxy Resins
2.1. Organic Solvent Liquefaction
2.2. Fast Pyrolysis
2.3. Hydrothermal Liquefaction
3. Injection of Sustainability into Epoxy Resin with Bio-Oils Produced from Thermochemical Processing
3.1. Synthesis of Epoxy Resin with Bio-Oil
3.2. Blending Bio-Oil with Conventional Petrochemical Epoxy Resins
4. Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- May, C.A. Epoxy Resins: Chemistry and Technology, 2nd ed.; Marcel Dekker: New York, NY, USA, 1988; p. 1. [Google Scholar]
- Bilyeu, B.; Brostow, W.; Menard, K.P. Epoxy thermosets and their applications I: Chemical structures and applications. J. Mater. Educ. 1999, 21, 281–286. [Google Scholar]
- Ueki, T.; Nishijima, S.; Izumi, Y. Designing of epoxy resin systems for cryogenic use. Cryogenics 2005, 45, 141–148. [Google Scholar] [CrossRef]
- Karnati, S.R.; Agbo, P.; Zhang, L. Applications of silica nanoparticles in glass/carbon fiber-reinforced epoxy nanocomposite. Compos. Commun. 2020, 17, 32–41. [Google Scholar] [CrossRef]
- Celikbag, Y.; Meadows, S.; Barde, M.; Adhikari, S.; Buschle-Diller, G.; Auad, M.L.; Via, B.K. Synthesis and characterization of bio-oil-based self-curing epoxy resin. Ind. Eng. Chem. Res. 2017, 56, 9389–9400. [Google Scholar] [CrossRef]
- Ellis, B. Introduction to the chemistry, synthesis, manufacture and characterization of epoxy resins. In Chemistry and Technology of Epoxy Resins; Springer Science + Business Media: Dordrecht, The Netherlands, 1993; pp. 1–36. [Google Scholar]
- Liu, J.-Q.; Bai, C.; Jia, D.-D.; Liu, W.-L.; He, F.-Y.; Liu, Q.-Z.; Yao, J.-S.; Wang, X.-Q.; Wu, Y.-Z. Design and fabrication of a novel superhydrophobic surface based on a copolymer of styrene and bisphenol A diglycidyl ether monoacrylate. RSC Adv. 2014, 4, 18025–18032. [Google Scholar] [CrossRef]
- Petrie, E.M. Epoxy Adhesive Formulations; McGraw-Hill: New York, NY, USA, 2006; pp. 1–26. [Google Scholar]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins; a review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P. Bisphenol A environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Investig. 2016, 39, 259–263. [Google Scholar] [CrossRef]
- Carroll, D.R.; Stone, R.B.; Sirignano, A.M.; Saindon, R.M.; Gose, S.C.; Friedman, M.A. Structural properties of recycled plastic/sawdust lumber decking planks. Resour. Conserv. Recycl. 2001, 31, 241–251. [Google Scholar] [CrossRef]
- Chabba, S.; Netravali, A. Composites get greener. Mater. Today 2003, 6, 22–29. [Google Scholar]
- Gandini, A.; Belgacem, M.N. The state of the art. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–16. [Google Scholar]
- Zhang, Q.; Philips, H.R.; Purchel, A.; Hexum, J.K.; Reineke, T.M. Sustainable and degradable epoxy resins from trehalose, cyclodextrin, and soybean oil yield tunable mechanical performance and cell adhesion. ACS Sustain. Chem. Eng. 2018, 6, 14967–14978. [Google Scholar] [CrossRef]
- Ortiz, P.; Vendamme, R.; Eevers, W. Fully biobased epoxy resins from fatty acids and lignin. Molecules 2020, 25, 1158. [Google Scholar] [CrossRef]
- Naik, N.; Shivamurthy, B.; Thimmappa, B.H.S.; Guo, Z.; Bhat, R. Bio-based epoxies: Mechanical characterization and their applicability in the development of eco-friendly composites. J. Compos. Sci. 2022, 6, 294. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R. Enabling green manufacture of polymer products via vegetable oil epoxides. Ind. Eng. Chem. Res. 2023, 62, 1725–1735. [Google Scholar] [CrossRef]
- Marriam, F.; Irshad, A.; Umer, I.; Asghar, M.A.; Atif, M. Vegetable oils as bio-based precursors for epoxies. Sustain. Chemsitry Pharm. 2023, 31, 100935. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.R.; Warwel, S. Complete and partial epoxidation of plant oils by lipase-catalyzed perhydrolysis. Ind. Crops Prod. 1999, 9, 125–132. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Lignin-based epoxy resins: Unravelling the relationship between structure and material properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef]
- Delmas, G.H.; Benjelloun-Mlayah, B.; Bigot, Y.L.; Delmas, M. Biolignin™ based epoxy resins. J. Appl. Polym. Sci. 2013, 127, 1863–1872. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Synthesis of lignin-based epoxy resins: Optimization of reaction parameters using response surface methodology. RSC Adv. 2014, 4, 31745–31753. [Google Scholar] [CrossRef]
- Xin, J.; Li, M.; Li, R.; Wolcott, M.P.; Zhang, J. Green epoxy resin system based on lignin and tung oil and its application in epoxy asphalt. ACS Sustain. Chem. Eng. 2016, 4, 2754–2761. [Google Scholar] [CrossRef]
- Rapi, Z.; Szolnoki, B.; Bako, P.; Niedermann, P.; Toldy, A.; Bodzay, B.; Keglevich, G.; Marosi, G. Synthesis and characterization of biobased epoxy monomers derived from D-glucose. Eur. Polym. J. 2015, 67, 375–382. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T.; Hassan, A. Recent advances in epoxy resin, natural fiber-reinforced epoxy composites and their applications. J. Reinf. Plast. Compos. 2016, 35, 447–470. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F., III. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent development of biobased epoxy resins: A review. Polym. -Plast. Technol. Eng. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Majid, R.A.; Mustapha, S.N.H. Vegetable oil-based epoxy resins and their composites with bio-based hardener: A short review. Polym. Plast. Technol. Mater. 2019, 58, 1311–1326. [Google Scholar] [CrossRef]
- Pappa, C.; Feghali, E.; Vanbroekhoven, K.; Triantafyllidis, K.S. Recent advances in epoxy resins and composites derived from lignin and related bio-oils. Curr. Opin. Green Sustain. Chem. 2022, 38, 100687. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil Production and Upgrading Research: A Review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Kishi, H.; Akamatsu, Y.; Noguchi, M.; Fujita, A.; Matsuda, S.; Nishida, H. Synthesis of epoxy resins from alcohol-liquefied wood and the mechanical properties of the cured resins. J. Appl. Polym. Sci. 2011, 120, 745–751. [Google Scholar] [CrossRef]
- Celikbag, Y.; Robinson, T.J.; Via, B.K.; Adhikari, S.; Auad, M.L. Pyrolysis oil substituted epoxy resin: Improved ratio optimization and crosslinking efficiency. J. Appl. Polym. Sci. 2015, 132, 42239. [Google Scholar] [CrossRef]
- Celikbag, Y.; Via, B.K.; Adhikari, S.; Wu, Y. Effect of liquefaction temperature on hydroxyl groups of bio-oil from loblolly pine (Pinus taeda). Bioresour. Technol. 2014, 169, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Ravindran, H.; Gupta, R.B.; Fasina, O.; Tu, M.; Fernando, S.D. Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour. Technol. 2010, 101, 8389–8395. [Google Scholar] [CrossRef] [PubMed]
- Celikbag, Y.; Via, B.K.; Adhikari, S.; Buschle-Diller, G.; Auad, M.L. The effect of ethanol on hydroxyl and carbonyl groups in biopolyol produced by hydrothermal liquefaction of loblolly pine: 31P-NMR and 19F-NMR analysis. Bioresour. Technol. 2016, 214, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pan, H. Synthesis of polymers from organic solvent liquefied biomass: A review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463. [Google Scholar] [CrossRef]
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Lange, J.-P. Lignocellulose liquefaction to biocrude: A tutorial review. ChemSusChem 2018, 11, 997–1014. [Google Scholar] [CrossRef]
- Liang, L.; Mao, Z.; Li, Y.; Wan, C.; Wang, T.; Zhang, L.; Zhang, L. Liquefaction of crop residues for polyol production. BioResources 2006, 1, 248–256. [Google Scholar] [CrossRef]
- Bridgwater, A.; Peacocke, G. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.-Z.; Zhu, X.-F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A.; Shirley, V.; Cheng, D. Hydrothermal pyrolysis of swine manure to bio-oil: Effects of operating parameters on products yield and characterization of bio-oil. J. Anal. Appl. Pyrolysis 2010, 88, 73–79. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly efficient liquefaction of woody biomass in hot-compressed alcohol- water co-solvents. Energy Fuels 2010, 24, 4659–4667. [Google Scholar] [CrossRef]
- Ogi, T.; Yokoyama, S.-Y.; Koguchi, K. Direct liquefaction of wood by alkali and alkaline earth salt in an aqueous phase. Chem. Lett. 1985, 14, 1199–1202. [Google Scholar] [CrossRef]
- Itoh, S.; Suzuki, A.; Nakamura, T.; Yokoyama, S.-Y. Production of heavy oil from sewage sludge by direct thermochemical liquefaction. Desalination 1994, 98, 127–133. [Google Scholar] [CrossRef]
- Minowa, T.; Murakami, M.; Dote, Y.; Ogi, T.; Yokoyama, S.-Y. Oil production from garbage by thermochemical liquefaction. Biomass Bioenergy 1995, 8, 117–120. [Google Scholar] [CrossRef]
- Suzuki, A.; Nakamura, T.; Yokoyama, S.-Y. Effect of operating parameters on thermochemical liquefaction of sewage sludge. J. Chem. Eng. Jpn. 1990, 23, 6–11. [Google Scholar] [CrossRef]
- Gharieb, H.K.; Faramawy, S.; Zaki, N. Liquefaction of cellulosic waste V. Water formation and evaluation of pyrolytic char as a by-product of pyrolysis reaction. Fuel Sci. Technol. Int. 1995, 13, 895–909. [Google Scholar] [CrossRef]
- He, B.J.; Zhang, Y.; Yin, Y.; Funk, T.L.; Riskowski, G.L. Operating temperature and retention time effects on the thermochemical conversion process of swine manure. Trans. ASAE 2000, 43, 1821. [Google Scholar]
- Balat, M. Mechanisms of thermochemical biomass conversion processes. Part 3: Reactions of liquefaction. Energy Sources Part A 2008, 30, 649–659. [Google Scholar] [CrossRef]
- He, B.J.; Zhang, Y.; Funk, T.L.; Riskowski, G.L.; Yin, Y. Thermochemical conversion of swine manure: An alternative process for waste treatment and renewable energy production. Trans. ASAE 2000, 43, 1827. [Google Scholar]
- Kishi, H.; Fujita, A.; Miyazaki, H.; Matsuda, S.; Murakami, A. Synthesis of wood-based epoxy resins and their mechanical and adhesive properties. J. Appl. Polym. Sci. 2006, 102, 2285–2292. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zhao, D. Synthesis of liquefied corn barn-based epoxy resins and their thermodynamic properties. J. Appl. Polym. Sci. 2012, 125, 2304–2311. [Google Scholar] [CrossRef]
- Wu, C.C.; Lee, W.J. Synthesis and properties of copolymer epoxy resins prepared from copolymerization of bisphenol A, epichlorohydrin, and liquefied Dendrocalamus latiflorus. J. Appl. Polym. Sci. 2010, 116, 2065–2073. [Google Scholar]
- Wu, C.C.; Lee, W.J. Curing and thermal properties of copolymer epoxy resins prepared by copolymerized bisphenol-A and epichlorohydrin with liquefied Dendrocalamus latiflorus. Polym. J. 2010, 42, 711–715. [Google Scholar] [CrossRef]
- Barde, M.; Adhikari, S.; Via, B.K.; Auad, M.L. Synthesis and characterization of epoxy resins from fast pyrolysis bio-oil. Green Mater. 2018, 6, 76–84. [Google Scholar] [CrossRef]
- Sibaja, B.; Adhikari, S.; Celikbag, Y.; Via, B.; Auad, M.L. Fast pyrolysis bio-oil as precursor of thermosetting epoxy resins. Polym. Eng. Sci. 2018, 58, 1296–1307. [Google Scholar] [CrossRef]
- Wu, C.C.; Lee, W.J. Curing behavior and adhesion properties of epoxy resin blended with polyhydric alcohol-liquefied Cryptomeria japonica wood. Wood Sci. Technol. 2011, 45, 559–571. [Google Scholar] [CrossRef]
- Wei, N.; Via, B.K.; Wang, Y.; McDonald, T.; Auad, M.L. Liquefaction and substitution of switchgrass (Panicum virgatum) based bio-oil into epoxy resins. Ind. Crops Prod. 2014, 57, 116–123. [Google Scholar] [CrossRef]
- Sorrentino, L.; Turchetta, S.; Parodo, G.; Papa, R.; Toto, E.; Santonicola, M.G.; Laurenzi, S. RIFT process analysis for the production of green composites in flax fibers and bio-based epoxy resin. Materials 2022, 15, 8173. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Wang, G.; Yu, D.; Kelkar, A.D.; Zhang, L. Electrospun nanofiber: Emerging reinforcing filler in polymer matrix composite materials. Prog. Polym. Sci. 2017, 75, 73–107. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Chen, C.; Justice, R.S.; Schaefer, D.W.; Baur, J.W. Highly dispersed nanosilica–epoxy resins with enhanced mechanical properties. Polymer 2008, 49, 3805–3815. [Google Scholar] [CrossRef]
- Wang, G.; Yu, D.; Mohan, R.V.; Gbewonyo, S.; Zhang, L. A comparative study of nanoscale glass filler reinforced epoxy composites: Electrospun nanofiber vs nanoparticle. Compos. Sci. Technol. 2016, 129, 19–29. [Google Scholar] [CrossRef]
- Haq, M.; Burgueno, R.; Mohanty, A.K.; Misra, M. Bio-based polymer nanocomposites from UPE/EML blends and nanoclay: Development, experimental characterization and limits to synergistic performance. Compos. Part A Appl. Sci. Manuf. 2011, 42, 41–49. [Google Scholar] [CrossRef]
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]





| Feedstock | Temperature (°C) | Pressure (MPa) | Time (s) | Yield of Oil (%) | Calorific Value (MJ/Kg) | Reference |
|---|---|---|---|---|---|---|
| Dairy Manure | 250–380 | 10–34 | – | 50 | – | [52] |
| Sewage Sludge | 300 | 10 | 30–1200 | 48 | 37–39 | [53] |
| Rubbish | 250–340 | 6–8 | 360–7200 | 27.6 | 36 | [54] |
| Sewage Sludge | 150–300 | 8–20 | 0–10,800 | 44.5 | 35.7 | [55] |
| Sewage Sludge | 250–350 | 8–20 | – | 30.7 | 36.4 | [49] |
| Municipal Solid Waste | 260–340 | 13–24 | – | 32 | 46 | [56] |
| Municipal Solid Waste | 295–450 | – | 1200–5400 | 35–63.3 | – | [57] |
| Sewage Sludge | 300–360 | 10–18 | 5–20 | – | 30–35 | [58] |
| Swine Manure | 305 | 10.3 | 80 | 70 | 25–33 | [59] |
| PEG400/Glycerin-Liquefied Wood-Based Epoxy Resin | PEG400/Glycerin Epoxy Resin | PEG200 Epoxy Resin | Bisphenol A-Type Epoxy Resin | |
|---|---|---|---|---|
| Tensile strength (MPa) | 27.9 | 1.4 | 3.5 | 62.8 |
| Yield stress (MPa) | 31.6 | - | - | - |
| Tensile modulus (GPa) | 1.27 | 0.04 | 0.01 | 2.06 |
| Lap-shear adhesive strength (MPa) | 14.5 | 1.5 | 1.7 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbo, P.; Mali, A.; Deng, D.; Zhang, L. Bio-Oil-Based Epoxy Resins from Thermochemical Processing of Sustainable Resources: A Short Review. J. Compos. Sci. 2023, 7, 374. https://doi.org/10.3390/jcs7090374
Agbo P, Mali A, Deng D, Zhang L. Bio-Oil-Based Epoxy Resins from Thermochemical Processing of Sustainable Resources: A Short Review. Journal of Composites Science. 2023; 7(9):374. https://doi.org/10.3390/jcs7090374
Chicago/Turabian StyleAgbo, Philip, Abhijeet Mali, Dongyang Deng, and Lifeng Zhang. 2023. "Bio-Oil-Based Epoxy Resins from Thermochemical Processing of Sustainable Resources: A Short Review" Journal of Composites Science 7, no. 9: 374. https://doi.org/10.3390/jcs7090374
APA StyleAgbo, P., Mali, A., Deng, D., & Zhang, L. (2023). Bio-Oil-Based Epoxy Resins from Thermochemical Processing of Sustainable Resources: A Short Review. Journal of Composites Science, 7(9), 374. https://doi.org/10.3390/jcs7090374