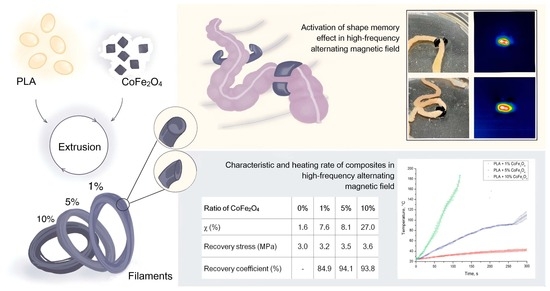
Impact of CoFe2O4 Magnetic Nanoparticles on the Physical and Mechanical Properties and Shape Memory Effect of Polylactide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of CoFe2O4 Nanoparticles (NPs)
2.2. Characterization of CoFe2O4 NPs
2.2.1. Transmission Electron Microscopy
2.2.2. X-ray Diffraction
2.2.3. Study of Magnetic Properties
2.3. Composite Material Preparation Technique
2.4. 3D Printing
2.5. Characterization of Composite Materials
2.5.1. Differential Scanning Calorimetry
2.5.2. Shape Memory Effect Parameters
2.5.3. Study of Mechanical Properties
2.5.4. SME Activation in a High-Frequency Alternating Magnetic Field
2.6. In Vitro Studies
2.6.1. MSC Isolation from Mice Adipose Tissue and Culture
2.6.2. MTS-Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Study of the Structure and Morphology of CoFe2O4 NPs
3.2. Study of the Magnetic Properties of CoFe2O4 NPs
3.3. Differential Scanning Calorimetry
3.4. Parameters of the Shape Memory Effect
3.5. Study of Mechanical Properties
3.6. SME Activation in a High-Frequency Alternating Magnetic Field
3.7. In Vitro Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bairwa, K.N.; Reddy, D.R. Influence of addition of Al2O3 and SiC on tensile and flexural characteristics of epoxy/glass fiber hybrid polymer composite. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tekay, E. Low-Voltage Triggered Electroactive and Heat-Responsive Thermoplastic Elastomer/Carbon Nanotube Polymer Blend Composites. Mater. Today Commun. 2023, 35, 106443. [Google Scholar] [CrossRef]
- Tang, X.; Pionteck, J.; Pötschke, P. Improved piezoresistive sensing behavior of poly (vinylidene fluoride)/carbon black composites by blending with a second polymer. Polymer 2023, 268, 125702. [Google Scholar] [CrossRef]
- Ismail, A.S.; Jawaid, M.; Hamid, N.H.; Yahaya, R.; Hassan, A.; Sarmin, S.N. Physical, structural and thermal properties of bio-phenolic/epoxy polymers blends. Mater. Today Commun. 2023, 34, 105455. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-responsive shape memory materials: A review. Mater. Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Smith, K.E.; Garcia, M.; Dupont, K.M.; Higgs, G.B.; Gall, K.; Safranski, D.L. Shape-memory Polymers for Orthopaedic Soft-Tissue Repair. Tech. Orthop. 2017, 32, 141–148. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Z.; Wu, D.; Chen, K.; Weng, S. Mechanics-guided design of inflatable heterogeneous shape memory polymer vascular stents. Int. J. Mech. Sci. 2023, 254, 108405. [Google Scholar] [CrossRef]
- Masuda, T.; Miyazawa, K.; Ueda, N.; Hata, Y.; Kawai, T.; Goto, S. Development of an orthodontic elastic material using EMA-based resin combined with 1-butanol. Dent. Mater. J. 2011, 30, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S.; Eppley, B.L.M. An experimental study of heat adaptation of bioabsorbable craniofacial meshes and plates. J. Craniofac. Surg. 2007, 18, 540–545. [Google Scholar] [CrossRef]
- Stiller, A.M.; Usoro, J.O.; Lawson, J.; Araya, B.; González-González, M.A.; Danda, V.R.; Voit, W.E.; Black, B.J.; Pancrazio, J.J. Mechanically robust, softening shape memory polymer probes for intracortical recording. Micromachines 2020, 11, 619. [Google Scholar] [CrossRef]
- Sánchez, C.P.; Jérôme, C.; Noels, L.; Vanderbemden, P. Review of Thermoresponsive Electroactive and Magnetoactive Shape Memory Polymer Nanocomposites. ACS Omega 2022, 7, 40701–40723. [Google Scholar] [CrossRef]
- Wu, L.; Jin, C.; Sun, X. Synthesis, properties, and light-induced shape memory effect of multiblock polyesterurethanes containing biodegradable segments and pendant cinnamamide groups. Biomacromolecules 2011, 12, 235–241. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.; Jünger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef]
- Lee, K.M.; Koerner, H.; Vaia, R.A.; Bunning, T.J.; White, T.J. Light-activated shape memory of glassy, azobenzene liquid crystalline polymer networks. Soft Matter 2011, 7, 4318–4324. [Google Scholar] [CrossRef]
- Ma, C.; Lu, W.; Yang, X.; He, J.; Le, X.; Wang, L.; Zhang, J.; Serpe, M.J.; Huang, Y.; Chen, T. Bioinspired anisotropic hydrogel actuators with on–off switchable and color-tunable fluorescence behaviors. Adv. Funct. Mater. 2018, 28, 1704568. [Google Scholar] [CrossRef]
- Liu, T.; Huang, R.; Qi, X.; Dong, P.; Fu, Q. Facile preparation of rapidly electro-active shape memory thermoplastic polyurethane/polylactide blends via phase morphology control and incorporation of conductive fillers. Polymer 2017, 114, 28–35. [Google Scholar] [CrossRef]
- Dong, Y.; Ni, Q.-Q.; Li, L.; Fu, Y. Novel vapor-grown carbon nanofiber/epoxy shape memory nanocomposites prepared via latex technology. Mater. Lett. 2014, 132, 206–209. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, L.; Bian, W.; Liu, Y.; Leng, J. 4D printed electro-induced continuous carbon fiber reinforced shape memory polymer composites with excellent bending resistance. Compos. Part B Eng. 2020, 194, 108034. [Google Scholar] [CrossRef]
- Wang, L.; Razzaq, M.Y.; Rudolph, T.; Heuchel, M.; Nöchel, U.; Mansfeld, U.; Jiang, Y.; Gould, O.E.C.; Behl, M.; Kratz, K.; et al. Reprogrammable, magnetically controlled polymeric nanocomposite actuators. Mater. Horiz. 2018, 5, 861–867. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wu, W.; Dong, X.; Sang, L. 4D printing of mechanically robust PLA/TPU/Fe3O4 magneto-responsive shape memory polymers for smart structures. Compos. Part B Eng. 2023, 248, 110382. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, G.; Xu, S.; Ma, T.; Nie, J. Biodegradable magnetic-sensitive shape memory poly (ε-caprolactone)/Fe3O4 nanocomposites. J. Appl. Polym. Sci. 2018, 135, 45652. [Google Scholar] [CrossRef]
- Du, H.; Liu, X.; Yu, Y.; Xu, Y.; Wang, Y.; Liang, Z. Microwave-Induced Poly(ionic liquid)/Poly(vinyl alcohol) Shape Memory Composites. Macromol. Chem. Phys. 2016, 217, 2626–2634. [Google Scholar] [CrossRef]
- Du, H.; Song, Z.; Wang, J.; Liang, Z.; Shen, Y.; You, F. Microwave-induced shape-memory effect of silicon carbide/poly(vinyl alcohol) composite. Sens. Actuators A Phys. 2015, 228, 1–8. [Google Scholar] [CrossRef]
- Correia, C.O.; Caridade, S.G.; Mano, J.F. Chitosan membranes exhibiting shape memory capability by the action of controlled hydration. Polymers 2014, 6, 1178–1186. [Google Scholar] [CrossRef]
- Yang, Y.; Terentjev, E.M.; Wei, Y.; Ji, Y. Solvent-assisted programming of flat polymer sheets into reconfigurable and self-healing 3D structures. Nat. Commun. 2018, 9, 1906. [Google Scholar] [CrossRef]
- Bhargava, A.; Peng, K.; Stieg, J.; Mirzaeifar, R.; Shahab, S. Focused ultrasound actuation of shape memory polymers; acoustic-thermoelastic modeling and testing. RSC Adv. 2017, 7, 45452–45469. [Google Scholar] [CrossRef]
- Li, G.; Yan, Q.; Xia, H.; Zhao, Y. Therapeutic-ultrasound-triggered shape memory of a melamine-enhanced poly(vinyl alcohol) physical hydrogel. ACS Appl. Mater. Interfaces 2015, 7, 12067–12073. [Google Scholar] [CrossRef]
- Garces, I.T.; Aslanzadeh, S.; Boluk, Y.; Ayranci, C. Effect of Moisture on shape memory polyurethane polymers for extrusion-based additive manufacturing. Materials 2019, 12, 244. [Google Scholar] [CrossRef]
- Li, S.-T.; Jin, X.-Z.; Shao, Y.-W.; Qi, X.-D.; Yang, J.-H.; Wang, Y. Gold nanoparticle/reduced graphene oxide hybrids for fast light-actuated shape memory polymers with enhanced photothermal conversion and mechanical stiffness. Eur. Polym. J. 2019, 116, 302–310. [Google Scholar] [CrossRef]
- Xie, H.; Shao, J.; Ma, Y.; Wang, J.; Huang, H.; Yang, N.; Wang, H.; Ruan, C.; Luo, Y.; Wang, Q.-Q.; et al. Biodegradable near-infrared-photoresponsive shape memory implants based on black phosphorus nanofillers. Biomaterials 2018, 164, 11–21. [Google Scholar] [CrossRef]
- Sun, L.; Wang, T.X.; Chen, H.M.; Salvekar, A.V.; Naveen, B.S.; Xu, Q.; Weng, Y.; Guo, X.; Chen, Y.; Huang, W.M. A brief review of the shape memory phenomena in polymers and their typical sensor applications. Polymers 2019, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Migneco, F.; Huang, Y.-C.; Birla, R.K.; Hollister, S.J. Poly(glycerol-dodecanoate), a biodegradable polyester for medical devices and tissue engineering scaffolds. Biomaterials 2009, 30, 6479–6484. [Google Scholar] [CrossRef]
- Garle, A.; Kong, S.; Ojha, U.; Budhlall, B.M. Thermoresponsive semicrystalline poly(ε-caprolactone) networks: Exploiting cross-linking with cinnamoyl moieties to design polymers with tunable shape memory. ACS Appl. Mater. Interfaces 2012, 4, 645–657. [Google Scholar] [CrossRef]
- Liu, Y.; Gall, K.; Dunn, M.L.; McCluskey, P. Thermomechanics of shape memory polymer nanocomposites. Mech. Mater. 2004, 36, 929–940. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandstroröm, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems: Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- García-García, G.; Lázaro-Callejón, M.; Fernández-Álvarez, F.; Iglesias, G.R.; Arias, J.L. (Magnetite/poly (ε-caprolactone))/chitosan (core/shell)/shell nanocomposites with potential applications in hyperthermia cancer therapy. J. Magn. Magn. Mater. 2023, 588, 171500. [Google Scholar] [CrossRef]
- Montazersaheb, P.; Pishgahzadeh, E.; Jahani, V.B.; Farahzadi, R.; Montazersaheb, S. Magnetic nanoparticle-based hyperthermia: A prospect in cancer stem cell tracking and therapy. Life Sci. 2023, 323, 121714. [Google Scholar] [CrossRef]
- Farzanegan, Z.; Tahmasbi, M. Evaluating the applications and effectiveness of magnetic nanoparticle-based hyperthermia for cancer treatment: A systematic review. Appl. Radiat. Isot. 2023, 198, 110873. [Google Scholar] [CrossRef]
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic hyperthermia: Potentials and limitations. J. Indian Chem. Soc. 2022, 99, 100269. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Zheng, Z.; Liu, Y.; Leng, J. Magnetic programming of 4D printed shape memory composite structures. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105571. [Google Scholar] [CrossRef]
- Kim, Y.; Yuk, H.; Zhao, R.; Chester, S.A.; Zhao, X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 2018, 558, 274–279. [Google Scholar] [CrossRef]
- Ma, C.; Wu, S.; Ze, Q.; Kuang, X.; Zhang, R.; Qi, H.J.; Zhao, R. Magnetic multimaterial printing for multimodal shape transformation with tunable properties and shiftable mechanical behaviors. ACS Appl. Mater. Interfaces 2020, 13, 12639–12648. [Google Scholar] [CrossRef]
- Schmidt, A.M. Electromagnetic Activation of Shape Memory Polymer Networks Containing Magnetic Nanoparticles. Macromol. Rapid Commun. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Integration of PCL and PLA in a monolithic porous scaffold for interface tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 63, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlchopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly (lactic Acid): A versatile biobased polymer for the future with multifunctional properties—From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, G.; Singh, S.; Jha, K.; Prakash, C. 3D printed biodegradable functional temperature-stimuli shape memory polymer for customized scaffoldings. J. Mech. Behav. Biomed. Mater. 2020, 108, 103781. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Senatov, F.S.; Niaza, N.K.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Hamad, K.; Ur Rehman, Z. Review of recent advances in polylactic acid/TiO2 composites. Materials 2019, 12, 3659. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F.; Ko, Y.G. A review on recent researches on polylactic acid/carbon nanotube composites. Polym. Bull. 2017, 74, 2921–2937. [Google Scholar] [CrossRef]
- Tsai, P.A.; Chiu, W.M.; Lin, C.E.; Wu, J.H. Fabrication and characterization of PLA/SiO2/Al2O3 composites prepared by Sol-Gel process. Polym. Plast. Technol. Eng. 2013, 52, 1488–1495. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Preparation and characterization of binary and ternary blends with poly (lactic acid), polystyrene, and acrylonitrile-butadiene-styrene. J. Biomater. Nanobiotechnol. 2012, 3, 405–412. [Google Scholar] [CrossRef]
- Zahn, D.; Landers, J.; Diegel, M.; Salamon, S.; Stihl, A.; Schacher, F.H.; Wende, H.; Dellith, J.; Dutz, S. Optimization of Magnetic Cobalt Ferrite Nanoparticles for Magnetic Heating Applications in Biomedical Technology. Nanomaterials 2023, 13, 1673. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef]
- Kharat, P.B.; Somvanshi, S.B.; Khirade, P.P.; Jadhav, K.M. Induction heating analysis of surface-functionalized nanoscale CoFe2O4 for magnetic fluid hyperthermia toward noninvasive cancer treatment. ACS Omega 2020, 5, 23378–23384. [Google Scholar] [CrossRef] [PubMed]
- Elbeshir, E.I.A. Magnetic and thermal properties of CoFe2O4 nanoparticles for magnetic hyperthermia treatment. Int. J. Adv. Appl. Scin. 2018, 5, 34–36. [Google Scholar] [CrossRef]
- Jia, W.; Qi, Y.; Hu, Z.; Xiong, Z.; Luo, Z.; Xiang, Z.; Hu, J.; Lu, W. Facile fabrication of monodisperse CoFe2O4 nanocrystals@dopamine@DOX hybrids for magnetic-responsive on-demand cancer theranostic applications. Adv. Compos. Hybrid Mater. 2021, 4, 989–1001. [Google Scholar] [CrossRef]
- Ashour, A.; El-Batal, A.I.; Maksoud, M.A.; El-Sayyad, G.S.; Labib, S.; Abdeltwab, E.; El-Okr, M. Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 2018, 40, 141–151. [Google Scholar] [CrossRef]
- De, D.; Upadhyay, P.; Das, A.; Ghosh, A.; Adhikary, A.; Goswami, M.M. Studies on cancer cell death through delivery of dopamine as anti-cancer drug by a newly functionalized cobalt ferrite nano-carrier. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127202. [Google Scholar] [CrossRef]
- Moeini, N.; Molaei, S.; Ghadermazi, M. Dysprosium (III) Supported on CoFe2O4 MNPs as a heterogeneous catalyst for the selective oxidation of sulfides and synthesis of symmetrical disulfides. J. Mol. Struct. 2021, 1246, 131071. [Google Scholar] [CrossRef]
- Garanina, A.S.; Nikitin, A.A.; Abakumova, T.O.; Semkina, A.S.; Prelovskaya, A.O.; Naumenko, V.A.; Erofeev, A.S.; Gorelkin, P.V.; Majouga, A.G.; Abakumov, M.A.; et al. Cobalt ferrite nanoparticles for tumor therapy: Effective heating versus possible toxicity. Nanomaterials 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Vallabani, N.V.S.; Singh, S.; Karakoti, A.S. Magnetic nanoparticles: Current trends and future aspects in diagnostics and nanomedicine. Curr. Drug Metab. 2019, 20, 457–472. [Google Scholar] [CrossRef]
- Kovaleva, P.A.; Pariy, I.O.; Chernozem, R.V.; Zadorozhnyy, M.Y.; Permyakova, E.S.; Kolesnikov, E.A.; Surmeneva, M.A.; Surmenev, R.A.; Senatov, F.S. Shape memory effect in hybrid polylactide-based polymer scaffolds functionalized with reduced graphene oxide for tissue engineering. Eur. Polym. J. 2022, 181, 111694. [Google Scholar] [CrossRef]
- Senatov, F.S.; Zadorozhnyy, M.Y.; Niaza, K.V.; Medvedev, V.V.; Kaloshkin, S.D.; Anisimova, N.Y.; Kiselevskiy, M.V.; Yang, K.-C. Shape memory effect in 3D-printed scaffolds for self-fitting implants. Eur. Polym. J. 2017, 93, 222–231. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-controlled magnetic nanoparticles hyperthermia inhibits primary tumor growth and metastases dissemination. Nanomed. Nanotechnol. Biol. Med. 2020, 25, 102171. [Google Scholar] [CrossRef] [PubMed]
- Battegazzore, D.; Bocchini, S.; Frache, A. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym. Lett. 2011, 5, 849–858. [Google Scholar] [CrossRef]
- Krassikova, L.S.; Karshieva, S.S.; Cheglakov, I.B.; Belyavsky, A.V. Combined treatment, based on lysomustine administration with mesenchymal stem cells expressing cytosine deaminase therapy, leads to pronounced murine Lewis lung carcinoma growth inhibition. J. Gene Med. 2016, 18, 220–233. [Google Scholar] [CrossRef]
- Vinosha, A.; Jeronsia, E.; Raja, K.; christina Fernandez, A.; Krishnan, S.; Das, J. Investigation of optical, electrical and magnetic properties of cobalt ferrite nanoparticles by naive co-precipitation technique. Optik 2016, 127, 9917–9925. [Google Scholar]
- Karaagac, O.; Yildiz, B.B.; Köçkar, H. The influence of synthesis parameters on one-step synthesized superparamagnetic cobalt ferrite nanoparticles with high saturation magnetization. J. Magn. Magn. Mater. 2019, 473, 262–267. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Ye, L.; Coates, P.; Caton-Rose, F. Multiple shape memory behavior of highly oriented long-chain-branched poly(lactic acid) and its recovery mechanism. J. Biomed. Mater. Res. Part A 2019, 107, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Yin, X.; Cai, Z.; Wang, J. Effect of crystallization on shape memory effect of poly(lactic acid). Polymers 2022, 14, 1569. [Google Scholar] [CrossRef] [PubMed]
- Ling, X. Thermal and X-ray Analysis on the Origin of Double Melting Phenomena of Poly (L-Lactic Acid) Films; The University of Tennessee: Knoxville, TN, USA, 2005. [Google Scholar]
- Gong, X.; Cheng, C.; Tang, C.Y.; Law, W.; Lin, X.; Chen, Y.; Chen, L.; Tsui, G.C.P.; Rao, N. Crystallization behavior of polylactide matrix under the influence of nano-magnetite. Polym. Eng. Sci. 2019, 59, 608–615. [Google Scholar] [CrossRef]







| CoFe2O4 Mass Fraction, % | Tg, °C | Tcc, °C | Tm, °C | χ |
|---|---|---|---|---|
| 0 | 58.4 | 123.5 | 169.2 | 1.6 |
| 1 | 62.7 | 108.4 | 172.9 | 7.6 |
| 5 | 62.2 | 103.8, 112.8 | 171.4 | 8.1 |
| 10 | 62.2 | 92.9, 107.8 | 168.4 | 27.0 |
| Weight Fraction of CoFe2O4, wt.% | 0 | 1 | 5 | 10 |
|---|---|---|---|---|
| Recovery stress variation, MPa | 3.0 | 3.2 | 3.5 | 3.6 |
| Activation temperature of SME, °C | 53.7 | 54.1 | 54.1 | 52.8 |
| Weight Fraction of CoFe2O4, wt.% | Elastic Modulus, MPa |
|---|---|
| 0 | 1201 ± 12 |
| 1 | 1205 ± 21 |
| 5 | 914 ± 97 |
| 10 | 760 ± 23 |
| Weight Fraction of CoFe2O4, wt.% | Recovery Coefficient, % |
|---|---|
| 1 | 84.89 ± 1.33 |
| 5 | 94.06 ± 0.47 |
| 10 | 93.82 ± 0.58 |
| Control | PLA | PLA/CoFe2O4 (1 wt.%) | PLA/CoFe2O4 (5 wt.%) | PLA/CoFe2O4 (10 wt.%) | |
|---|---|---|---|---|---|
| Proportion of viable cells (mean value), % | 100.0 ± 4.8 | 89.4 ± 9.6 | 93.5 ± 6.4 | 92.1 ± 11.1 | 87.0 ± 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimina, A.; Nikitin, A.; Lvov, V.; Bulygina, I.; Kovaleva, P.; Vodopyanov, S.; Zadorozhnyy, M.; Peshkina, E.; Karshieva, S.; Choudhary, R.; et al. Impact of CoFe2O4 Magnetic Nanoparticles on the Physical and Mechanical Properties and Shape Memory Effect of Polylactide. J. Compos. Sci. 2024, 8, 48. https://doi.org/10.3390/jcs8020048
Zimina A, Nikitin A, Lvov V, Bulygina I, Kovaleva P, Vodopyanov S, Zadorozhnyy M, Peshkina E, Karshieva S, Choudhary R, et al. Impact of CoFe2O4 Magnetic Nanoparticles on the Physical and Mechanical Properties and Shape Memory Effect of Polylactide. Journal of Composites Science. 2024; 8(2):48. https://doi.org/10.3390/jcs8020048
Chicago/Turabian StyleZimina, Anna, Aleksey Nikitin, Vladislav Lvov, Inna Bulygina, Polina Kovaleva, Stepan Vodopyanov, Mikhail Zadorozhnyy, Elizaveta Peshkina, Saida Karshieva, Rajan Choudhary, and et al. 2024. "Impact of CoFe2O4 Magnetic Nanoparticles on the Physical and Mechanical Properties and Shape Memory Effect of Polylactide" Journal of Composites Science 8, no. 2: 48. https://doi.org/10.3390/jcs8020048
APA StyleZimina, A., Nikitin, A., Lvov, V., Bulygina, I., Kovaleva, P., Vodopyanov, S., Zadorozhnyy, M., Peshkina, E., Karshieva, S., Choudhary, R., Abakumov, M., & Senatov, F. (2024). Impact of CoFe2O4 Magnetic Nanoparticles on the Physical and Mechanical Properties and Shape Memory Effect of Polylactide. Journal of Composites Science, 8(2), 48. https://doi.org/10.3390/jcs8020048