A Comprehensive Study of Structural, Thermal, and Dielectric Properties of Melt-Processed Polypropylene/Ni0.9Zn0.1Fe2O4 Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
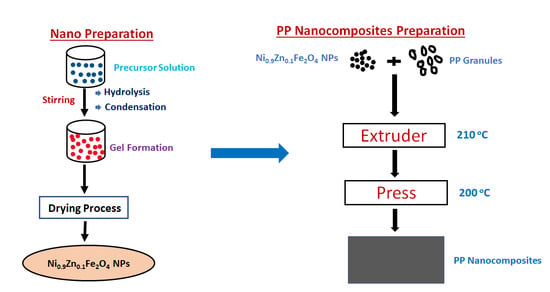
2.2. Sample Preparation and Characterization Techniques
3. Results
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. TEM Microscopy
3.4. FESEM Microscopy
3.5. Thermogravimetric Analysis
3.6. Dielectric Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Badawi, A. Characterization of the optical and mechanical properties of CdSe QDs/PMMA nanocomposite films. J. Mater. Sci. Mater. Electron. 2015, 26, 3450–3457. [Google Scholar] [CrossRef]
- Al-Osaimi, J.; Al-Hosiny, N.; Abdallah, S.; Badawi, A. Characterization of optical, thermal and electrical properties of SWCNTs/PMMA nanocomposite films. Iran. Polym. J. 2014, 23, 437–443. [Google Scholar] [CrossRef]
- Guo, M.; Jiang, J.; Shen, Z.; Lin, Y.; Nan, C.W.; Shen, Y. High-energy-density ferroelectric polymer nanocomposites for capacitive energy storage: Enhanced breakdown strength and improved discharge efficiency. Mater. Today 2019, 29, 49–67. [Google Scholar] [CrossRef]
- Donya, H.; Taha, T.A.; Alruwaili, A.; Tomsah, I.B.I.; Ibrahim, M. Micro structure and optical spectroscopy of PVA/iron oxide polymer nanocomposites. J. Mater. Res. Technol. 2020, 9, 9189–9194. [Google Scholar] [CrossRef]
- Taha, T.A.M.; Alanazi SSSEl-Nasser, K.; Alshammari, A.H.; Ismael, A. Structure–Property Relationships in PVDF/SrTiO3/CNT Nanocomposites for Optoelectronic and Solar Cell Applications. Polymers 2024, 16, 736. [Google Scholar] [CrossRef]
- Taha, T.A.; Mahmoud, M.H.; Hamdeh, H.H. Development, thermal and dielectric investigations of PVDF-Y2O3 polymer nanocomposite films. J. Polym. Res. 2021, 28, 148. [Google Scholar] [CrossRef]
- Taha, T.A.; Alzara, M.A.A. Synthesis, thermal and dielectric performance of PVA-SrTiO3 polymer nanocomposites. J. Mol. Struct. 2021, 1238, 130401. [Google Scholar] [CrossRef]
- Alhassan, S.; Alshammari, K.; Alshammari, M.; Alotaibi, T.; Alshammari, A.H.; Alhamazani, A.; Taha, T.A.M. Linear and nonlinear optical investigations of polyvinyl chloride modified La2O3 nanocomposite films. Results Phys. 2024, 58, 107456. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alhassan, S.; Iraqi, A.; Saad, S.A.; Taha, T.A.M. Dielectric relaxation investigations of polyester/CoFe2O4 composites. J. Mater. Sci. Mater. Electron. 2023, 34, 2132. [Google Scholar] [CrossRef]
- Kim, W.S.; Song, H.S.; Lee, B.O.; Kwon, K.H.; Lim, Y.S.; Kim, M.S. Electrical properties of PVDF/PVP composite filled with carbon nanotubes prepared by floating catalyst method. Macromol. Res. 2002, 10, 253–258. [Google Scholar] [CrossRef]
- Barber, P.; Balasubramanian, S.; Anguchamy, Y.; Gong, S.; Wibowo, A.; Gao, H.; Ploehn, H.J.; Zur Loye, H.C. Polymer composite and nanocomposite dielectric materials for pulse power energy storage. Materials 2009, 2, 1697–1733. [Google Scholar] [CrossRef]
- Li, D.G.; Chen, C.; Rao, W.; Lu, W.H.; Xiong, Y.H. Preparation and microwave absorption properties of polyaniline/Mn0.8Zn0.2Fe2O4 nanocomposite in 2–18 GHz. J. Mater. Sci. Mater. Electron. 2014, 25, 76–81. [Google Scholar] [CrossRef]
- Fang, X.; Ma, J.; Gu, C.; Xiong, W.; Jiang, T. Synchronous enhancement of electromagnetic and chemical effects-induced quantitative adsorptive detection of quercetin based on flexible polymer-silver-ZIF-67 SERS substrate. Sens. Actuators B Chem. 2023, 378, 133176. [Google Scholar] [CrossRef]
- Song, F.; Shen, X.; Liu, M.; Xiang, J. Preparation and magnetic properties of SrFe12O19/Ni0.5Zn0.5Fe2O4 nanocomposite ferrite microfibers via sol–gel process. Mater. Chem. Phys. 2011, 126, 791–796. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Abdel-Kader, M.H. Effect of excess oxygen content within different nano-oxide additives on the structural and optical properties of PVA/PEG blend. Appl. Phys. A 2019, 125, 209. [Google Scholar] [CrossRef]
- Nangia, R.; Shukla, N.K.; Sharma, A. Optical and structural properties of Se80Te15Bi5/PVA nanocomposite films. J. Mol. Struct. 2019, 1177, 323–330. [Google Scholar] [CrossRef]
- Soliman, T.S.; Vshivkov, S.A. Effect of Fe nanoparticles on the structure and optical properties of polyvinyl alcohol nanocomposite films. J. Non-Cryst. Solids 2019, 519, 119452. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R.J. Investigation on structural and dielectric properties of silica nanoparticles incorporated poly (ethylene oxide)/poly (vinyl pyrrolidone) blend matrix based nanocomposites. J. Inorg. Organomet. Polym. Mater. 2019, 29, 592–607. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J. Recent development on modification of synthesized barium titanate (BaTiO3) and polymer/BaTiO3 dielectric composites. J. Mater. Sci. Mater. Electron. 2019, 30, 1957–1975. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hassan, A.Q.; Mohammed, S.J.; Karim, W.O.; Kadir, M.F.Z.; Tajuddin, H.A.; Chan, N.N.M.Y. Structural and optical characteristics of PVA: C-Dot composites: Tuning the absorption of ultraviolet (UV) region. Nanomaterials 2019, 9, 216. [Google Scholar] [CrossRef]
- Nawar, A.M.; Mohammed, M.I.; Yahia, I.S. Facile synthesis and optical characterization of graphene oxide-doped TiO2/polyvinyl alcohol nanocomposites: Optical limiting applications. Mater. Res. Express 2019, 6, 075054. [Google Scholar] [CrossRef]
- Stepanov, A.L. Optical properties of polymer nanocomposites with functionalized nanoparticles. In Polymer Composites with Functionalized Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–355. [Google Scholar]
- Banerjee, M.; Jain, A.; Mukherjee, G.S. Microstructural and optical properties of polyvinyl alcohol/manganese chloride composite film. Polym. Compos. 2019, 40, E765–E775. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying poly(vinyl alcohol) (PVA) from insulator to small-bandgap polymer: A novel approach for organic solar cells and optoelectronic devices. J. Electron. Mater. 2016, 45, 736–745. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, H.; Sun, X.; Wang, X. Combination of cobalt ferrite and graphene: High-performance and recyclable visible-light photocatalysis. Appl. Catal. B Environ. 2012, 111, 280–287. [Google Scholar] [CrossRef]
- Sankaranarayanan, V.K.; Pankhurst, Q.A.; Dickson, D.P.E.; Johnson, C.E. An investigation of particle size effects in ultrafine barium ferrite. J. Magn. Magn. Mater. 1993, 125, 199–208. [Google Scholar] [CrossRef]
- Sankaranarayanan, V.K.; Gajbhiye, N.S. Low-Temperature Preparation of Ultrafine Rare-Earth Iron Garnets. J. Am. Ceram. Soc. 1990, 73, 1301–3007. [Google Scholar] [CrossRef]
- Virden, A.E.; O’Grady, K. Structure and magnetic properties of NiZn ferrite nanoparticles. J. Magn. Magn. Mater. 2005, 290, 868–870. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.A.; Yamaguchi, M. Radio frequency characteristics of Fe-filled carbon nanotube film. J. Magn. Magn. Mater. 2006, 302, 232–236. [Google Scholar] [CrossRef]
- Kubade, P.; Tambe, P. Influence of halloysite nanotubes (HNTs) on morphology, crystallization, mechanical and thermal behaviour of PP/ABS blends and its composites in presence and absence of dual compatibilizer. Compos. Interfaces 2016, 23, 433–451. [Google Scholar] [CrossRef]
- Kubade, P.; Tambe, P. Influence of surface modification of halloysite nanotubes and its localization in PP phase on mechanical and thermal properties of PP/ABS blends. Compos. Interfaces 2017, 24, 469–487. [Google Scholar] [CrossRef]
- Tambe, P.; Sharma, A.; Kulkarni, H.; Panda, B. Influence of surface treated halloysite nanotubes on mechanical and thermal properties of polypropylene nanocomposites. Mater. Today Proc. 2022, 56, 1351–1355. [Google Scholar] [CrossRef]
- Lohar, G.; Tambe, P.; Jogi, B. Influence of dual compatibilizer and carbon black on mechanical and thermal properties of PP/ABS blends and their composites. Compos. Interfaces 2020, 27, 1101–1136. [Google Scholar] [CrossRef]
- Jogi, B.F.; Lohar, G.; Pankaj, T. Influence of carbon black (CB) on 80/20 (wt/wt) poly-propylene (PP)/acrylonitrile-butadiene-styrene (ABS) nanocomposites: Localization, morphological behavior, crystallinity and thermal stability. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; Volume 2341, p. 040014. [Google Scholar]
- Patil, J.; Patil, H.; Sankpal, R.; Rathod, D.; Patil, K.; Kubade, P.R.; Kulkarni, H.B. Studies on mechanical and thermal performance of carbon nanotubes/polypropylene nanocomposites. Mater. Today Proc. 2021, 46, 7182–7186. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Manickam, S.; Ratnam, C.T.; Raghu, M.S.; Parashuram, L.; Prashantha, K.; Jeon, B.H. Surface-treated short sisal fibers and halloysite nanotubes for synergistically enhanced performance of polypropylene hybrid composites. J. Thermoplast. Compos. Mater. 2020, 35, 2089–2104. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Li, S. Review of electrical properties for polypropylene based nanocomposite. Compos. Commun. 2018, 10, 221–225. [Google Scholar] [CrossRef]
- Furlan, L.G.; Ferreira, C.I.; Dal Castel, C.; Santos, K.S.; Mello, A.C.E.; Liberman, S.A.; Oviedo, M.A.S.; Mauler, R.S. Effect of processing conditions on the mechanical and thermal properties of high-impact polypropylene nanocomposites. Mater. Sci. Eng. A 2011, 528, 6715–6718. [Google Scholar] [CrossRef]
- Uyor, U.O.; Popoola, A.P.I.; Popoola, O.M.; Aigbodion, V.S. Thermal, mechanical and dielectric properties of functionalized sandwich BN-BaTiO3-BN/polypropylene nanocomposites. J. Alloys Compd. 2022, 894, 162405. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Baloch, H.A.; Nizamuddin, S.; Kashi, S.; Tanjung, F.A.; Hossain, N.; Mazari, S.A.; Mubarak, N.M.; Griffin, G.J.; Srinivasan, M. Thermal, mechanical, rheological, electrical and electromagnetic interference shielding performance of polypropylene/magnetic carbon nanocomposites. J. Environ. Chem. Eng. 2021, 9, 105447. [Google Scholar] [CrossRef]
- Sonawane, S.; Thakur, P.; Paul, R. Study on thermal property enhancement of MWCNT based polypropylene (PP) nanocomposites. Mater. Today Proc. 2020, 27, 550–555. [Google Scholar] [CrossRef]
- Rishaban, R.; Balakrishnan, G.; Hema, R. Synthesis and properties of polypropylene-graphene nanocomposite. Mater. Today Proc. 2022, 62, 1977–1983. [Google Scholar] [CrossRef]
- Bendaoued, A.; Messaoud, M.; Harzallah, O.; Bistac, S.; Salhi, R. Nano-TiO2 effect on thermal, rheological and structural properties of thermoplastic polypropylene nanocomposites. J. Mater. Res. Technol. 2022, 17, 2313–2325. [Google Scholar] [CrossRef]
- Mandal, D.K.; Bhunia, H.; Bajpai, P.K.; Chaudhari, C.V.; Dubey, K.A.; Varshney, L.; Kumar, A. Preparation and characterization of polypropylene/polylactide blends and nanocomposites and their biodegradation study. J. Thermoplast. Compos. Mater. 2021, 34, 725–744. [Google Scholar] [CrossRef]
- Tambe, P.B.; Bhattacharyya, A.R.; Kamath, S.S.; Kulkarni, A.R.; Sreekumar, T.V.; Srivastav, A.; Rao, K.B.; Liu, Y.; Kumar, S. Structure–property relationship studies in amine functionalized multiwall carbon nanotubes filled polypropylene composite fiber. Polym. Eng. Sci. 2012, 52, 1183–1194. [Google Scholar] [CrossRef]
- Tambe, P.B.; Bhattacharyya, A.R.; Kulkarni, A.R. The influence of melt-mixing process conditions on electrical conductivity of polypropylene/multiwall carbon nanotubes composites. J. Appl. Polym. Sci. 2013, 127, 1017–1026. [Google Scholar] [CrossRef]
- Lohar, G.; Tambe, P.; Jogi, B. Migration induced localization of multiwalled carbon nanotubes in PP phase of PP/ABS blend and their hybrid composites: Influence on mechanical and thermal properties. Mater. Today Proc. 2022, 49, 1215–1224. [Google Scholar] [CrossRef]
- Yeole, P.; Alwekar, S.; Veluswamy, N.K.P.; Kore, S.; Hiremath, N.; Vaidya, U.; Theodore, M. Characterization of textile-grade carbon fiber polypropylene composites. Polym. Polym. Compos. 2021, 29, 652–659. [Google Scholar] [CrossRef]
- Alzebdeh, K.I.; Nassar, M.M.; Arunachalam, R. Effect of fabrication parameters on strength of natural fiber polypropylene composites: Statistical assessment. Measurement 2019, 146, 195–207. [Google Scholar] [CrossRef]
- Pak, S.; Park, S.; Song, Y.S.; Lee, D. Micromechanical and dynamic mechanical analyses for characterizing improved interfacial strength of maleic anhydride compatibilized basalt fiber/polypropylene composites. Compos. Struct. 2018, 193, 73–79. [Google Scholar] [CrossRef]
- Wang, D.; Xuan, L.; Han, G.; Wong, A.H.; Wang, Q.; Cheng, W. Preparation and characterization of foamed wheat straw fiber/polypropylene composites based on modified nano-TiO2 particles. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105674. [Google Scholar] [CrossRef]
- Awad, S.A.; Khalaf, E.M. Investigation of improvement of properties of polypropylene modified by nano silica composites. Compos. Commun. 2019, 12, 59–63. [Google Scholar] [CrossRef]
- Liang, J.Z. Effects of tension rates and filler size on tensile properties of polypropylene/graphene nano-platelets composites. Compos. Part B Eng. 2019, 167, 241–249. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Grząbka-Zasadzińska, A.; Borysiak, S.; Jesionowski, T. Preparation and characterization of polypropylene composites reinforced by functional ZnO/lignin hybrid materials. Polym. Test. 2019, 79, 106058. [Google Scholar] [CrossRef]
- Sivakumar, N.; Narayanasamy, A.; Ponpandian, N.; Greneche, J.M.; Shinoda, K.; Jeyadevan, B.; Tohji, K. Effect of mechanical milling on the electrical and magnetic properties of nanostructured Ni0.5Zn0.5Fe2O4. J. Phys. D Appl. Phys. 2006, 39, 4688. [Google Scholar] [CrossRef]
- Astafyev, A.; Lysenko, E.; Surzhikov, A.; Nikolaev, E.; Vlasov, V. Thermomagnetometric analysis of nickel–zinc ferrites. J. Therm. Anal. Calorim. 2020, 142, 1775–1781. [Google Scholar] [CrossRef]
- Kumbhar, S.S.; Mahadik, M.A.; Mohite, V.S.; Rajpure, K.Y.; Kim, J.H.; Moholkar, A.V.; Bhosale, C.H. Structural, dielectric and magnetic properties of Ni substituted zinc ferrite. J. Magn. Magn. Mater. 2014, 363, 114–120. [Google Scholar] [CrossRef]
- Taha, T.A.; Elrabaie, S.; Attia, M.T. Green synthesis, structural, magnetic, and dielectric characterization of NiZnFe2O4/C nanocomposite. J. Mater. Sci. Mater. Electron. 2018, 29, 18493–18501. [Google Scholar] [CrossRef]
- Machado, G.; Denardin, E.L.G.; Kinast, E.J.; Gonçalves, M.C.; De Luca, M.A.; Teixeira, S.R.; Samios, D. Crystalline properties and morphological changes in plastically deformed isotatic polypropylene evaluated by X-ray diffraction and transmission electron microscopy. Eur. Polym. J. 2005, 41, 129–138. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Microcrystalline-cellulose and polypropylene based composite: A simple, selective and effective material for microwavable packaging. Carbohydr. Polym. 2016, 142, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Akinci, A.; Akbulut, H.; Yilmaz, F. The effect of the red mud on polymer crystallization and the interaction between the polymer-filler. Polym.-Plast. Technol. Eng. 2007, 46, 31–36. [Google Scholar] [CrossRef]
- Mahrous, N.; Motawie, A.M.; Hakim, A.E.A.; Eid, A.I.; El-Sawey, A.A.A.-M. Study of some polypropylene nanocomposite properties. Egypt. J. Chem. 2018, 61, 825–842. [Google Scholar] [CrossRef]
- Taha, T.A.; Elrabaie, S.; Attia, M.T. Exploring the structural, thermal and dielectric properties of PVA/Ni0.5Zn0.5Fe2O4 composites. J. Electron. Mater. 2019, 48, 6797–6806. [Google Scholar] [CrossRef]
- Taha, T.A.; Hassona, A.; Elrabaie, S.; Attia, M.T. Micro-structure, thermal, and dielectric performance of polyester nanocomposites containing nano-Ni0.5Zn0.5Fe2O4. Appl. Phys. A 2020, 126, 761. [Google Scholar] [CrossRef]
- Saito, T.; Okamoto, M. Polypropylene-based nano-composite formation: Delamination of organically modified layered filler via solid-state processing. Polymer 2010, 51, 4238–4242. [Google Scholar] [CrossRef]
- Bhuiyan, M.K.H.; Rahman, M.M.; Mina, M.F.; Islam, M.R.; Gafur, M.A.; Begum, A. Crystalline morphology and properties of multi-walled carbon nanotube filled isotactic polypropylene nanocomposites: Influence of filler size and loading. Compos. Part A Appl. Sci. Manuf. 2013, 52, 70–79. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless melt-electrospinning of polypropylene nanofibres. J. Nanomater. 2012, 2012, 382639. [Google Scholar] [CrossRef]
- Hedrick, S.A.; Chuang, S.S. Temperature programmed decomposition of polypropylene: In situ FTIR coupled with mass spectroscopy study. Thermochim. Acta 1998, 315, 159–168. [Google Scholar] [CrossRef]
- Abdel-Hamid, H.M. Effect of electron beam irradiation on polypropylene films—Dielectric and FT-IR studies. Solid-State Electron. 2005, 49, 1163–1167. [Google Scholar] [CrossRef]
- Manova, E.; Kunev, B.; Paneva, D.; Mitov, I.; Petrov, L.; Estournès, C.; D’Orléans, C.; Rehspringer, J.-L.; Kurmoo, M. Mechano-synthesis, characterization, and magnetic properties of nanoparticles of cobalt ferrite, CoFe2O4. Chem. Mater. 2004, 16, 5689–5696. [Google Scholar] [CrossRef]
- Taha, T.A. Optical properties of PVC/Al2O3 nanocomposite films. Polym. Bull. 2019, 76, 903–918. [Google Scholar] [CrossRef]
- Taha, T.A.; Ismail, Z.; Elhawary, M.M. Structural, optical and thermal characterization of PVC/SnO2 nanocomposites. Appl. Phys. A 2018, 124, 307. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M.H. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans. Nonferrous Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]
- Gaabour, L.H. Thermal spectroscopy and kinetic studies of PEO/PVDF loaded by carbon nanotubes. J. Mater. 2015, 2015, 824859. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X. A thermal degradation mechanism of polyvinyl alcohol/silica nanocomposites. Polym. Degrad. Stab. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Diefallah, E.H.M. Kinetic analysis of thermal decomposition reactions: Part VI. Thermal decomposition of manganese (II) acetate tetrahydrate. Thermochim. Acta 1992, 202, 1–16. [Google Scholar] [CrossRef]
- Chetehouna, K.; Belayachi, N.; Rengel, B.; Hoxha, D.; Gillard, P. Investigation on the thermal degradation and kinetic parameters of innovative insulation materials using TGA-MS. Appl. Therm. Eng. 2015, 81, 177–184. [Google Scholar] [CrossRef]
- Vlaev, L.; Nedelchev, N.; Gyurova, K.; Zagorcheva, M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J. Anal. Appl. Pyrolysis 2008, 81, 253–262. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Grabchev, I.; Petkov, C.; Bojinov, V. Infrared spectral characterization of poly (amidoamine) dendrimers peripherally modified with 1,8-naphthalimides. Dye. Pigment. 2004, 62, 229–234. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70, 487. [Google Scholar] [CrossRef]
- Tomer, V.; Polizos, G.; Randall, C.A.; Manias, E. Polyethylene nanocomposite dielectrics: Implications of nanofiller orientation on high field properties and energy storage. J. Appl. Phys. 2011, 109, 074113. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; Ai, T.; Wu, K.; Zhao, F.; Gu, H. A novel fiber-reinforced polyethylene composite with added silicon nitride particles for enhanced thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2009, 40, 830–836. [Google Scholar] [CrossRef]
- Mansour, S.A.; Elsad, R.A.; Izzularab, M.A. Dielectric investigation of high-density polyethylene loaded by ZnO nanoparticles synthesized by sol–gel route. J. Sol-Gel Sci. Technol. 2016, 80, 333–341. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Mansour, S.A.; Elsad, R.A.; Izzularab, M.A. Dielectric properties enhancement of PVC nanodielectrics based on synthesized ZnO nanoparticles. J. Polym. Res. 2016, 23, 85. [Google Scholar] [CrossRef]
- Roy, M.; Nelson, J.K.; MacCrone, R.K.; Schadler, L.S.; Reed, C.W.; Keefe, R. Polymer nanocomposite dielectrics-the role of the interface. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 629–643. [Google Scholar] [CrossRef]
- Couderc, H.; David, E.; Frechette, M. Study of water diffusion in PE-SiO2 nanocomposites by dielectric spectroscopy. Trans. Electr. Electron. Mater. 2014, 15, 291–296. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Du, F.Y.; Liu, X.; Lv, J.H.; He, L.; Li, J.L.; Zhou, Y.L. Optimizing the dielectric constant of the shell layer in core–shell structures for enhanced energy density of polymer nanocomposites. Phys. Chem. Chem. Phys. 2023, 25, 18030–18037. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, E.; Sauers, I.; James, D.R.; Ellis, A.R.; Paranthaman, M.P.; Aytuğ, T.; Sathyamurthy, S.; More, K.L.; Li, J.; Goyal, A. Electrical properties of epoxy resin based nano-composites. Nanotechnology 2006, 18, 025703. [Google Scholar] [CrossRef]
- Ciuprina, F.; Plesa, I.; Notingher, P.V.; Tudorache, T.; Panaitescu, D. Dielectric properties of nanodielectrics with inorganic fillers. In Proceedings of the 2008 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Quebec, QC, Canada, 26–29 October 2008; IEEE: New York, NY, USA, 2008; pp. 682–685. [Google Scholar]
- Taha, T.A.; Hendawy, N.; El-Rabaie, S.; Esmat, A.; El-Mansy, M.K. Fluorescence and dielectric spectroscopy identification of polyvinyl chloride/NiO nanocomposites. J. Mol. Struct. 2020, 1212, 128162. [Google Scholar] [CrossRef]
- Amor, I.B.; Rekik, H.; Kaddami, H.; Raihane, M.; Arous, M.; Kallel, A. Studies of dielectric relaxation in natural fiber–polymer composites. J. Electrost. 2009, 67, 717–722. [Google Scholar] [CrossRef]
- Giuntini, J.C.; Zanchetta, J.V.; Jullien, D.; Eholie, R.; Houenou, P. Temperature dependence of dielectric losses in chalcogenide glasses. J. Non-Cryst. Solids 1981, 45, 57–62. [Google Scholar] [CrossRef]
- Song, Y.; Shen, Y.; Liu, H.; Lin, Y.; Li, M.; Nan, C.W. Improving the dielectric constants and breakdown strength of polymer composites: Effects of the shape of the BaTiO3 nanoinclusions, surface modification and polymer matrix. J. Mater. Chem. 2012, 22, 16491–16498. [Google Scholar] [CrossRef]
- Usmanov, S.M.; Zaikov, G.E. Numerical Methods of Solving Ill-Posed Problems of Dielectric Spectrometry; Nova Publishers: Hauppauge, NY, USA, 2002. [Google Scholar]
- Abouhaswa, A.S.; Taha, T.A. Tailoring the optical and dielectric properties of PVC/CuO nanocomposites. Polym. Bull. 2020, 77, 6005–6016. [Google Scholar] [CrossRef]
- Shukla, N.; Thakur, A.K.; Shukla, A.; Chatterjee, R. Dielectric relaxation and thermal studies on dispersed phase polymer nanocomposite films. J. Mater. Sci. Mater. Electron. 2014, 25, 2759–2770. [Google Scholar] [CrossRef]
- Sadiq, M.; Arya, A.; Ali, J.; Singh, N.P.; Sharma, A.L. Electrical conductivity and dielectric properties of solid polymer nanocomposite films: Effect of BaTiO3 nanofiller. Mater. Today Proc. 2020, 32, 476–482. [Google Scholar] [CrossRef]
- Wang, Q.; Che, J.; Wu, W.; Hu, Z.; Liu, X.; Ren, T.; Zhang, J. Contributing Factors of Dielectric Properties for Polymer Matrix Composites. Polymers 2023, 15, 590. [Google Scholar] [CrossRef]












| Sample ID | Polypropylene (wt%) | Ni0.9Zn0.1Fe2O4 (wt%) |
|---|---|---|
| PP | 100 | 0.0 |
| PP-NZF5 | 95 | 5.0 |
| PP-NZF10 | 90 | 10 |
| PP-NZF15 | 85 | 15 |
| Ni0.9Zn0.1Fe2O4 NPs Percentage (wt%) | Degradation Stage | Mass Loss (%) | |||||
|---|---|---|---|---|---|---|---|
| ∆T (°C) | Tpeak (°C) | Mass Loss (%) | ∆ m250 | ∆ m350 | ∆ m450 | ∆ m550 | |
| 0 | 353–594 | 490 | 99.64 | 0.17 | 0.44 | 22.05 | 99.58 |
| 5 | 311–594 | 467 | 97.00 | 0.66 | 2.36 | 44.27 | 97.04 |
| 10 | 342–596 | 498 | 94.89 | 0.69 | 1.94 | 8.3 | 94.83 |
| 15 | 322–596 | 499 | 92.38 | 0.96 | 2.65 | 9.07 | 92.27 |
| Ni0.9Zn0.1Fe2O4 NPs Percentage (wt%) | Ea (KJ/mol) | A (S−1) | ΔS* (J/mol.K) | ΔH* (KJ/mol) | ΔG* (KJ/mol) |
|---|---|---|---|---|---|
| 0 | 393.83 | 2.64 × 1011 | −38.70 | 389.75 | 408.71 |
| 5 | 315.83 | 2.55 × 109 | −76.90 | 311.95 | 347.89 |
| 10 | 264.67 | 1.04 × 107 | −123.18 | 260.53 | 321.90 |
| 15 | 381.55 | 1.41 × 1011 | −44.08 | 377.40 | 399.39 |
| Ni0.9Zn0.1Fe2O4 NPs Percentage (wt.%) | εs | ε∞ | Δε | τo (µs) | fo (kHz) | Wm (eV) |
|---|---|---|---|---|---|---|
| 0 | 1.52 | 1.26 | 0.26 | 24.2 | 6.58 | 0.106 |
| 5 | 2.13 | 2.09 | 0.04 | 5.42 | 29.36 | 0.068 |
| 10 | 2.58 | 2.49 | 0.09 | 0.99 | 160.76 | 0.119 |
| 15 | 1.84 | 1.76 | 0.08 | 1.05 | 151.58 | 0.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, T.A.M.; Tharwat, M.; Ismael, A. A Comprehensive Study of Structural, Thermal, and Dielectric Properties of Melt-Processed Polypropylene/Ni0.9Zn0.1Fe2O4 Nanocomposites. J. Compos. Sci. 2024, 8, 117. https://doi.org/10.3390/jcs8040117
Taha TAM, Tharwat M, Ismael A. A Comprehensive Study of Structural, Thermal, and Dielectric Properties of Melt-Processed Polypropylene/Ni0.9Zn0.1Fe2O4 Nanocomposites. Journal of Composites Science. 2024; 8(4):117. https://doi.org/10.3390/jcs8040117
Chicago/Turabian StyleTaha, Taha Abdel Mohaymen, Mohamed Tharwat, and Ali Ismael. 2024. "A Comprehensive Study of Structural, Thermal, and Dielectric Properties of Melt-Processed Polypropylene/Ni0.9Zn0.1Fe2O4 Nanocomposites" Journal of Composites Science 8, no. 4: 117. https://doi.org/10.3390/jcs8040117
APA StyleTaha, T. A. M., Tharwat, M., & Ismael, A. (2024). A Comprehensive Study of Structural, Thermal, and Dielectric Properties of Melt-Processed Polypropylene/Ni0.9Zn0.1Fe2O4 Nanocomposites. Journal of Composites Science, 8(4), 117. https://doi.org/10.3390/jcs8040117