Remarkable Separation of Carbofuran Pesticide from Aqueous Solution Using Free Metal Ion Variation on Aluminum-Based Metal-Organic Framework
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of MIL-53-NH2
2.3. Post-Synthetic Modification of MIL-53-NH2
2.3.1. Synthesis of MIL-53-NH-ph
2.3.2. Synthesis of MIL-53-NH-ph-M (M = Fe, Cu, Zn)
2.4. Characterization of the Synthesized Compounds
2.5. Adsorption Study
3. Results and Discussion
3.1. Metal Complex Inside the Nanopores of MIL-53-NH2
3.2. Characterization
3.2.1. 1H NMR of MIL-53-NH-Ph
3.2.2. IR of the Synthesized Compounds
3.2.3. XRD and SEM analysis
3.3. Adsorption Analysis Study
3.3.1. Effect of pH on the Adsorption Process
3.3.2. Effect of Pesticide Concentration
3.3.3. Effect of Time on the Adsorption Process
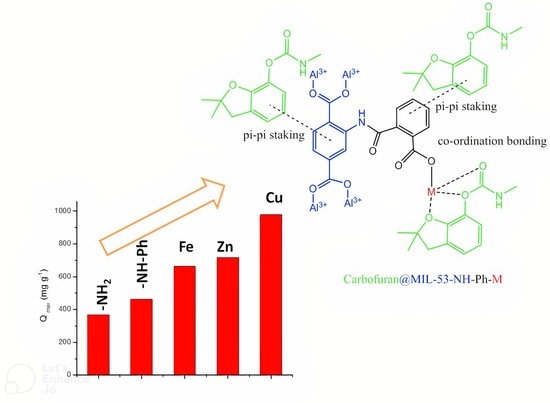
3.4. Adsorption Mechanism
3.5. Comparison of the Obtained Results with Previous Work
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Kamel, F.; Hoppin, J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004, 112, 950–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.C.; Goad, J.T.; Kadel, W.L. Cholinergic and noncholinergic changes in skeletal muscles by carbofuran and methyl parathion. J. Toxicol. Environ. Health Part A Curr. Issues 1994, 43, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Otieno, P.O.; Lalah, J.O.; Virani, M.; Jondiko, I.O.; Schramm, K.-W. Soil and water contamination with carbofuran residues in agricultural farmlands in Kenya following the application of the technical formulation Furadan. J. Environ. Sci. Health Part B 2010, 45, 137–144. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Patil, D.V.; Rallapalli, P.B.S.; Dangi, G.P.; Tayade, R.J.; Somani, R.S.; Bajaj, H.C. MIL-53 (Al): An efficient adsorbent for the removal of nitrobenzene from aqueous solutions. Ind. Eng. Chem. Res. 2011, 50, 10516–10524. [Google Scholar] [CrossRef]
- Tang, J.; Ma, X.; Yang, J.; Feng, D.-D.; Wang, X.-Q. Recent advances in metal–organic frameworks for pesticide detection and adsorption. Dalton Trans. 2020, 49, 14361–14372. [Google Scholar] [CrossRef]
- Abdelhameed, R.; Abdel-Gawad, H.; Silva, C.; Rocha, J.; Hegazi, B.; Silva, A. Kinetic and equilibrium studies on the removal of 14 C-ethion residues from wastewater by copper-based metal–organic framework. Int. J. Environ. Sci. Technol. 2018, 15, 2283–2294. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Taha, M.; Abdel-Gawad, H.; Mahdy, F.; Hegazi, B. Zeolitic imidazolate frameworks: Experimental and molecular simulation studies for efficient capture of pesticides from wastewater. J. Environ. Chem. Eng. 2019, 7, 103499. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Emam, H.E. Macroporous Cu-MOF@ cellulose acetate membrane serviceable in selective removal of dimethoate pesticide from wastewater. J. Environ. Chem. Eng. 2021, 9, 105121. [Google Scholar] [CrossRef]
- Mirsoleimani-azizi, S.M.; Setoodeh, P.; Samimi, F.; Shadmehr, J.; Hamedi, N.; Rahimpour, M.R. Diazinon removal from aqueous media by mesoporous MIL-101(Cr) in a continuous fixed-bed system. J. Environ. Chem. Eng. 2018, 6, 4653–4664. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Yang, J.; Li, Y.; Zhao, W.; Shi, J.; Gu, J. Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl. Mater. Interfaces 2015, 7, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.M.; Taha, M.; Abdel-Gawad, H.; Hegazi, B. Amino-functionalized Al-MIL-53 for dimethoate pesticide removal from wastewater and their intermolecular interactions. J. Mol. Liq. 2021, 327, 114852. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Elshahat, M.; Emam, H.E. Cu–BTC@ cotton composite: Design and removal of ethion insecticide from water. RSC Adv. 2016, 6, 42324–42333. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Herzog, R. Kapillarchemie, eine Darstellung der Chemie der Kolloide und Verwandter Gebiete. von dr. Herbert Freundlich. Verlag der Akademischen Verlagsgesellschaft; Wiley Online Library: Leipzig, Germany, 1909. [Google Scholar]
- Rodrigues, A.E.; Silva, C.M. What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem. Eng. J. 2016, 306, 1138–1142. [Google Scholar] [CrossRef]
- Krishna, K.R.; Philip, L. Adsorption and desorption characteristics of lindane, carbofuran and methyl parathion on various Indian soils. J. Hazard. Mater. 2008, 160, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Vimal, V.; Patel, M.; Mohan, D. Aqueous carbofuran removal using slow pyrolyzed sugarcane bagasse biochar: Equilibrium and fixed-bed studies. RSC Adv. 2019, 9, 26338–26350. [Google Scholar] [CrossRef] [Green Version]
- Roudani, A.; Mamouni, R.; Saffaj, N.; Laknifli, A.; Gharby, S.; Faouzi, A. Removal of carbofuran pesticide from aqueous solution by adsorption onto animal bone meal as new low cost adsorbent. Chem. Process Eng. Res. 2014, 28, 2014. [Google Scholar]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Salman, J.; Hameed, B. Adsorption of 2, 4-dichlorophenoxyacetic acid and carbofuran pesticides onto granular activated carbon. Desalination 2010, 256, 129–135. [Google Scholar] [CrossRef]
- Salman, J.; Abd, F.; Muhammed, A. Adsorption of carbofuran insecticide from aqueous solution using commercial activated carbon. Int. J. Chem. Sci. 2011, 9, 557–564. [Google Scholar]
- Salman, J.; Njoku, V.; Hameed, B. Bentazon and carbofuran adsorption onto date seed activated carbon: Kinetics and equilibrium. Chem. Eng. J. 2011, 173, 361–368. [Google Scholar] [CrossRef]
- Salman, J.M. Batch study for insecticide carbofuran adsorption onto palm-oil-fronds-activated carbon. J. Chem. 2013, 2013, 630371. [Google Scholar] [CrossRef] [Green Version]
- Toledo-Jaldin, H.P.; Sánchez-Mendieta, V.; Blanco-Flores, A.; López-Téllez, G.; Vilchis-Nestor, A.R.; Martín-Hernández, O. Low-cost sugarcane bagasse and peanut shell magnetic-composites applied in the removal of carbofuran and iprodione pesticides. Environ. Sci. Pollut. Res. 2020, 27, 7872–7885. [Google Scholar] [CrossRef]
- Chang, K.-L.; Lin, J.-H.; Chen, S.-T. Adsorption studies on the removal of pesticides (Carbofuran) using activated carbon from rice straw agricultural waste. Int. J. Agric. Biosyst. Eng. 2011, 5, 210–213. [Google Scholar]
- Chang, K.-L.; Chen, C.-C.; Lin, J.-H.; Hsien, J.-F.; Wang, Y.; Zhao, F.; Shih, Y.-H.; Xing, Z.-j.; Chen, S.-T. Rice straw-derived activated carbons for the removal of carbofuran from an aqueous solution. New Carbon Mater. 2014, 29, 47–54. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saini, V.K. Adsorption of 2, 4-D and carbofuran pesticides using fertilizer and steel industry wastes. J. Colloid Interface Sci. 2006, 299, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-q.; Hu, Z.-j.; Ji, R. Removal of carbofuran from aqueous solution by orange peel. Desalination Water Treat. 2012, 49, 106–114. [Google Scholar] [CrossRef]











| Pesticide | MOFs | Surface Area (m2 g−1) | pH | Adsorption Capacity (mol g−1) | Reference |
|---|---|---|---|---|---|
| Diazinon | MIL-101 (Cr) | 2600 | 7 | 0.855 | [12] |
| Ethion | Cu-BTC | 980 | 7 | 0.317 | [9] |
| Prothiofos | ZIF-8 (Zn) | 1800 | 7 | 1.07 | [11] |
| Ethion | ZIF-8 (Zn) | 1800 | 7 | 0.726 | [10] |
| Prothiofos | ZIF-67 (Co) | 1250 | 7 | 0.761 | [10] |
| Ethion | ZIF-67 (Co) | 1250 | 7 | 0.549 | [10] |
| Glyphosate | UIO-67 (Zr) | 2172 | 4 | 3.18 | [13] |
| Dimethoate | Al-MOF | 1260 | 7 | 2.24 | [14] |
| Ethion | Cu-BTC@cotton | 750 | 7 | 0.474 | [15] |
| Dimethoate | Cu-BTC@ cellulose acetate | 965.8 | 7 | 1.694 | [11] |
| Samples | Elemental Analysis (%) * | Surface Area (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | Al | Zn | Fe | Cu | ||||
| MIl-53-NH2 | 43.9 (43.07) | 2.55 (2.71) | 6.33 (6.28) | 12.3 (12.09) | - | - | - | 1060 | 0.8 | 0.5 |
| MIL-53-NH-ph | 48.51 (48.9) | 2.78 (2.71) | 4.75 (4.6) | 8.91 (8.86) | - | - | - | 950 | 0.7 | 0.47 |
| MIL-53-NH-ph-Fe | 44.98 (44.49) | 2.31 (2.30) | 4.25 (4.18) | 8.28 (8.06) | - | 9.5 (9.18) | - | 750 | 0.5 | 0.42 |
| MIL-53-NH-ph-Zn | 43.91 (43.81) | 2.14 (2.27) | 4.31 (4.12) | 8.1 (7.94) | 10.3 (10.5) | - | - | 725 | 0.44 | 0.41 |
| MIL-53-NH-ph-Cu | 44.1 (43.94) | 2.32 (2.27) | 4.12 (4.13) | 8.15 (7.96) | - | - | 10.5 (10.3) | 730 | 0.47 | 0.44 |
| Samples | Freundlich Parameters | Langmuir Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| n | kF (mg g−1) (mL mg−1)1/n | R2 | χ2 | Qm (mg g−1) | kL (mL mg−1) | R2 | χ2 | |
| MIL-53-NH2 | 2.27 ± 0.09 | 76.08 ± 4.15 | 0.937 | 444.8 | 367.87 ± 4.58 | 0.173 ± 0.04 | 0.986 | 21.07 |
| MIL-53-NH-Ph | 2.14 ± 0.12 | 81.88 ± 5.96 | 0.949 | 167.7 | 462.06 ± 2.83 | 0.127 ± 0.014 | 0.992 | 18.04 |
| MIL-53-NH-Ph-Fe | 1.86 ± 0.33 | 124.00 ± 3.64 | 0.927 | 4322 | 662.94 ± 2.75 | 0.117 ± 0.037 | 0.958 | 85.19 |
| MIL-53-NH-Ph-Zn | 3.61 ± 0.86 | 304.92 ± 5.21 | 0.938 | 5216 | 717.62 ± 6.71 | 0.068 ± 0.09 | 0.995 | 36.03 |
| MIL-53-NH-Ph-Cu | 2.97 ± 0.98 | 370.27 ± 10.21 | 0.771 | 3427 | 978.64 ± 9.76 | 0.048 ± 0.24 | 0.988 | 14.34 |
| Samples | Qe Exp. (mg g−1) | Pseudo-First-Order Parameters | Pseudo-Second-Order Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (mg g−1) | k1 (min−1) | R2 | χ2 | Qe | k2 × 10−5 (g mg−1 min−1) | R2 | χ2 | ||
| MIL-53-NH2 | 309 | 431.66 ± 12.92 | 0.0143 ± 0.001 | 0.926 | 370.6 | 315.42 ± 4.67 | 1.88 ±0.02 | 0.997 | 28.99 |
| MIL-53-NH-Ph | 366 | 557.28 ± 26.83 | 0.0186 ± 0.003 | 0.938 | 270.1 | 369.11 ± 2.76 | 0.14 ± 0.02 | 0.992 | 49.04 |
| MIL-53-NH-Ph-Fe | 616 | 809.12 ± 32.21 | 0.0270 ± 0.001 | 0.946 | 145.3 | 619.76 ± 8.91 | 1.54 ± 0.17 | 0.997 | 10.91 |
| MIL-53-NH-Ph-Zn | 689 | 974.29 ± 34.54 | 0.0430 ± 0.002 | 0.966 | 201.1 | 673.56 ± 8.27 | 6.07 ± 1.16 | 0.986 | 16.12 |
| MIL-53-NH-Ph-Cu | 976 | 1041.4 ± 36.31 | 0.0492 ± 0.001 | 0.957 | 256.6 | 978.34 ± 4.89 | 1.88 ± 0.18 | 0.996 | 39.76 |
| Adsorbent | pH | Qm (mg g−1) | Reference |
|---|---|---|---|
| MIL-53-NH-Ph-Cu | 7 | 978.6 | Current work |
| MIL-53-NH-Ph-Fe | 7 | 717.6 | Current work |
| MIL-53-NH-Ph-Zn | 7 | 662.9 | Current work |
| MIL-53-NH-Ph | 7 | 462.1 | Current work |
| MIL-53-NH2 | 7 | 367.8 | Current work |
| Indian soils | 7 | 0.9–4.9 | [19] |
| Slow pyrolyzed sugarcane bagasse biochar | 6 | 3.6–18.9 | [20] |
| Animal bone meal | 6 | 18.5 | [21] |
| Tea waste biochars | 5 | 22.8–54.7 | [22] |
| Granular activated carbon | 7 | 96.2 | [23] |
| Commercial activated carbon | 7 | 97.1 | [24] |
| Date seed-activated carbon | 5.5 | 137.02 | [25] |
| Palm-oil-fronds-activated carbon | 7 | 164.0 | [26] |
| Magnetic sugarcane bagasse | 7 | 175.0 | [27] |
| Activated carbon from rice straw | 7 | 296.5 | [28] |
| Rice straw-derived activated carbon | 7 | 222.2–312.5 | [29] |
| Steel (blast furnace slag dust and sludge) | 7.5 | 208 | [30] |
| Orange peel | 7 | 84.49 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabil, M.; Elantabli, F.M.; El-Medani, S.M.; Abdelhameed, R.M. Remarkable Separation of Carbofuran Pesticide from Aqueous Solution Using Free Metal Ion Variation on Aluminum-Based Metal-Organic Framework. Colloids Interfaces 2022, 6, 73. https://doi.org/10.3390/colloids6040073
Nabil M, Elantabli FM, El-Medani SM, Abdelhameed RM. Remarkable Separation of Carbofuran Pesticide from Aqueous Solution Using Free Metal Ion Variation on Aluminum-Based Metal-Organic Framework. Colloids and Interfaces. 2022; 6(4):73. https://doi.org/10.3390/colloids6040073
Chicago/Turabian StyleNabil, Marwa, Fatma M. Elantabli, Samir M. El-Medani, and Reda M. Abdelhameed. 2022. "Remarkable Separation of Carbofuran Pesticide from Aqueous Solution Using Free Metal Ion Variation on Aluminum-Based Metal-Organic Framework" Colloids and Interfaces 6, no. 4: 73. https://doi.org/10.3390/colloids6040073
APA StyleNabil, M., Elantabli, F. M., El-Medani, S. M., & Abdelhameed, R. M. (2022). Remarkable Separation of Carbofuran Pesticide from Aqueous Solution Using Free Metal Ion Variation on Aluminum-Based Metal-Organic Framework. Colloids and Interfaces, 6(4), 73. https://doi.org/10.3390/colloids6040073