1. Introduction
Four-antennary oligoglycines ([Gly
n-NHCH
2]
4C, T4) are synthetic substances with specific self-assembling performance in aqueous environments [
1,
2]. Due to the presence of equal-length oligopeptide tails attached to a central carbon atom, they can build up disk-like supramolecular entities—
tectomers. The key driving force for the onset of these nanostructures is the predisposition of T4 molecules to form extended intra- and intermolecular H-bonding networks at high cooperativity levels arranged in Polyglycine II motifs (PG-II, helix-3
1) [
3,
4,
5]. Because of the four-tail bonding to a central C atom, appropriate mutual orientations of CO and NH groups in the neighboring antennae within a single oligoglycine molecule are possible, which result in the onset of intramolecular helix-3
1 motifs. This inherent PG-II tendency generates the inception of intramolecular “click-clack” configurations and creates a specific
-conformation of the single T4 molecule [
1,
2]. These primarily structured species serve as building blocks for the further development of the tectomeric self-assemblies. These are based on the consecutive association of
-formed T4 units via intermolecular H-bonds into hexagonal packing of disk-like supramolecular entities, characterized by unusual stability [
1]. This phenomenon is closely related to the number of the glycine units in the antennae: there must be at least 7 (n ≥ 7) so as to fulfill the demands of configuring the effective “click-clack” network of highly coordinated intra- and intermolecular helix-3
1 motifs [
1].
In fluid environments, the onset of PG-II tendencies is coupled with auxiliary interaction options involving the other components of the system. For example, in aqueous solutions and at ambient conditions, portions of NH
2 terminal groups are charged to NH
3+ [
1,
2,
6], and both the single oligoglycine molecules and the tectomers are positively charged in water. Hence, complementary fine-tuning possibilities for changes in the structure–property relationships arise, e.g., through variations in pH, the addition of low-molecular-mass (LMM) electrolytes, and the insertion of negatively charged ingredients. The interplay of the PG-II-formation tendencies and the aqueous solution composition provides considerable application potential for solutions containing four-antennary oligopeptides [
2,
6]. An essential option has been identified recently: in the presence of traces of bacterial lipopolysaccharides (LPS, endotoxins), T4+LPS complexes are formed so as to effectively capture and block the endotoxins in an aqueous environment [
6,
7,
8].
LPSs build the external cover layer of the cell walls of Gram-negative bacteria like the human pathogen
Escherichia coli (
E. coli) [
9,
10]. When separated from the cell wall, LPSs remain toxic, with the lethal concentrations for humans being very low [
11,
12,
13,
14]. While there are methods to capture LPSs in aqueous media, they are either too specific or refer to higher quantities of the endotoxin (e.g., [
13,
14,
15]). The development of new approaches, particularly those aimed at the identification of ultralow quantities of LPSs, are constantly needed, and one such approach was proposed in our earlier studies [
6,
7,
8]. It is based on the fact that LPS molecules are amphiphilic substances: as key structural components, they have hydrocarbon chains (Lipid A) and a core polysaccharide region. In particular, the presence of phosphate groups in the core portions is responsible for the electric (negative) charge of the LPS in an aqueous environment. Due to electrostatic interactions with the T4 supramolecular entities in water solutions, two major types of T4+LPS complexes appear: amphiphilic species with the LPS molecules attached to one site of the tectomers, and “sandwich-like” hydrophilic T4+LPS entities, with LPS in between two discotic T4 self-assemblies [
6,
8]. At sufficient T4 content in the in aqueous milieu, these phenomena result in complete entrapment of the LPS traces. In addition, the onset of the T4+LPS complexes and their sizes may be additionally regulated via changes in the pH of the systems at ambient temperatures [
8]. An important unsolved problem remains, however: how to remove the entrapped LPS from the aqueous solutions. This issue is particularly important in cases where super-clean water is necessary, e.g., in hemodialysis, neonatology, etc.
The aim of the present study is to implement systematic investigations of the influence of temperature variations on the different options for removal of trace LPS quantities captured by the T4 tectomers. One additional goal is also to verify the possibility to develop consecutive recovering of the extra T4 quantities that did not participate in the formation of T4+LPS complexes. This research concerns both bulk solution structure–property performance and adsorption-layer enactment as a result of moderate changes in temperature in aqueous systems.
2. Materials and Methods
Four-antennary oligoglycine (T4) was purchased from PlasmaChem GmbH (Berlin, Germany) in a salt form ([Gly
7-NHCH
2]
4C*4HCl, purity 95%). Lipopolysaccharides from
Escherichia coli EH100 (LPSs) were supplied by Sigma Aldrich (Ra-mutant, purity ≥ 97%). Doubly distilled water (2D water) was used for the preparation of the aqueous samples. The T4 solutions were initially incubated for 24 h. The oligoglycine concentration was 2 × 10
−4 mol/L. The LPS solutions were prepared with an initial concentration of 5 µg/L and stored at 4 °C for at least one month. The investigated fluid systems were obtained from the LPS stock solution and the final endotoxin concentration in all measurements was 0.5 µg/L. The specific choice of the concentrations corresponds to the optimal T4 quantity for capturing the whole LPS quantity at the “native pH” (pH = 5.70 at 20 °C), as established in [
8].
Britton–Robinson universal buffer was applied to maintain the pH of the samples [
16]. The buffer was composed of boric acid (H
3BO
3, Sigma Aldrich, St. Louis, MO, USA, purity ≥ 99.97% (trace metal basis)), phosphoric acid (H
3PO
4, Sigma Aldrich, purity ≥ 99.999% (trace metal basis)), and acetic acid (CH
3COOH, Sigma Aldrich, purity ≥ 99.99% (trace metal basis)) in the ratio of 1:1:1 by volume. The components were mixed with sodium hydroxide (Sigma Aldrich, NaOH, purity ≥ 97% (titration using HCl)) so as to obtain a fixed pH = 6.2.
The adsorption layer properties at the air–solution interface were investigated by means of the emerging-bubble option of profile analysis tensiometry (PAT-1, Sinterface, Berlin, Germany) [
17,
18,
19]. The measurements were implemented in the course of about 48 h. The surface dilational rheology was studied through oscillations at low frequencies of 0.005–0.2 Hz; the oscillation amplitudes were in the range of 5% of the bubble surface area. The experiments were carried out at various temperatures in between 20.0 ± 0.1 and 85.0 ± 0.1 °C, with the samples kept either in the thermostatic chamber of the PAT instrumentation or in another thermostatic device between the separate measurements.
Some test examinations of the bulk solution properties were performed via dynamic light sScattering (DLS, Zetasizer Nano ZS, Malvern Instruments Ltd., Malvern, UK). The scattering angle was 173° (non-invasive backwards scattering). The aggregate size distributions were recalculated using Mie theory [
20,
21].
Two types of systems were investigated:
(1) Aqueous solutions of T4+LPS, prepared with doubly distilled water (2D water), at “native pH”, without specific pH regulation (pH = 5.7 at 20 °C). This procedure included initial thermal equilibration for 20 min. The temperature was further increased stepwise from 20 °C to 60 °C at ten-degree intervals, keeping the system for the respective thermal equilibration in the course of 1 h at all the thermal steps. The surface dilational rheology was investigated thereafter using the same bubble. Finally, the system was left at 60 °C in the course of 7 h, then subjected to a sharp cool-down procedure back to 20 °C.
(2) Aqueous solutions of T4+LPS, and of T4 only, prepared with Britton–Robinson universal buffer, at specific pH and ambient temperature (pH = 6.2 at 20 °C). These values were chosen because in previous studies it was established that at these conditions, the whole quantity of the LPS traces in the system is evidenced to be captured by the tectomers [
8]. The system was exposed to a ten-degree step-wise heating procedure, the same as in case (1). The solution was kept for 7 h at 60 °C, thereafter followed by a slow cooling process, with one-hour steps down to 20 °C. The latter temperature was maintained for the course of 3.5 h, and the last cooling temperature step was down to 10 °C, which was also kept constant for 3.5 h. Another temperature heating–cooling cycle was performed in the range of 20 °C to 85 °C, with various times of incubation at 85 °C (30 min, 1 h, 4 h), followed by either a fast or slow (24 h) cooling procedure down to 20 °C.
4. Discussion
The obtained results might be summarized as follows:
1. The combined heating-and-cooling cycle results are dependent on the pH of the aqueous solutions.
At “native pH” (pH = 5.7), the initial (20 °C, at the start of the heating procedure) surface-tension values are recovered upon proceeding back to 20 °C, while their time evolution differs: there is a visible “lag-period” in the case without heating and a sharp decrease upon cooling the heated sample (
Figure 2a). However, at the end of the heating–cooling cycle, the surface-dilational elasticities are systematically lower compared to the respective data for the unheated samples (
Figure 2b). The results might be interpreted as being due to an onset of structural reorganizations in the sub-interfacial bulk region that are still not so advanced enough as to have an impact on the equilibrium surface tension of the samples.
At the end of the heating–cooling procedure at controlled pH = 6.2, lower surface tensions (
Figure 3 and
Figure 5a) and higher surface dilational elasticities (
Figure 4a and
Figure 5b) are achieved. The higher the temperatures reached upon heating the sample, the lower the final surface tension values obtained at the end of the heating–cooling cycle (20 °C). The data strongly evidence that upon temperature variations, the structural characteristics, both in the subsurface bulk phase and at the adsorption layer at the air–solution interface, are irreversibly changed. In addition, the results in
Figure 4a,b evidence that in the heating branch, the possible structural changes are mainly in the solution bulk, while in the cooling branch, we do already have a depletion of the amphiphilic species, most probably due to their adsorption at the air–solution interface within the time of keeping the system at 60 °C.
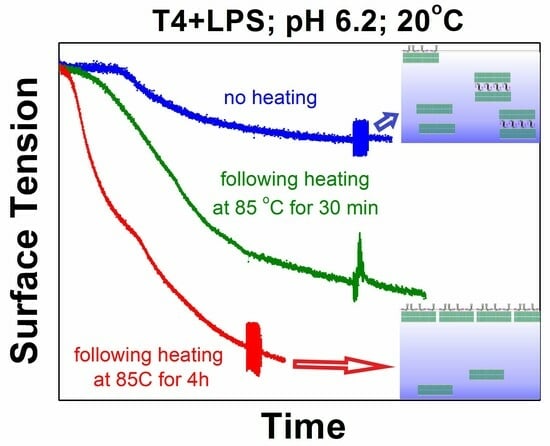
2. The runs of the surface tension data at controlled pH = 6.2 and 20 °C are very sensitive to the time variations in preliminarily keeping the system at a particular high temperature (
Figure 5a). The longer incubation time of the samples at high temperature results in a sharper decrease in the surface tension values and lower final data when cooled to 20 °C. The respective changes in the surface dilational elasticities are even more affected by both the temperature changes and the duration of keeping the samples at high temperatures (
Figure 5b). These facts also indicate that the acquired surface tension values and the surface rheological data are actually quasi-stationary and depend on the incubation time at the specific system’s conditions. The detailed procedure of the temperature-decrease route and the onset of the well-outlined hysteresis loop additionally demonstrate a clear coupling between the kinetics of structural reorganization in the investigated systems and the temperature variations (
Figure 3).
The obtained experimental outcomes give grounds for the advancement of the following hypothesis:
Due to the specific interrelationships between the PG-II tendencies and the components of the surrounding aqueous environment, the structural evolution of the two types of T4+LPS complexes, namely amphiphilic and “sandwich-like” entities, can be effectively regulated through temperature variations and the duration of the incubation times at the highest temperature values. The data evidence that upon heating, the sandwich-like species are “opened”/destroyed, becoming amphiphilic species (
Scheme 1). This is fully in line with classical concepts about temperature-variation effects on hydrophobic interactions [
23,
24]. Upon heating the samples, the impact of thermal regulation results in the weakening of the H-bonded network in aqueous solution. Thus, the “sandwich”-type T4+LPS complexes that are formed because of hydrophobic interactions LPS+LPS at low temperature (20 °C) are “loosened” at higher temperatures (60 °C and 85 °C) and turned into amphiphilic T4+LPS complexes. Due to the innate amphiphilicity of the LPS molecules, these T4+LPS species are surface-active and effectively adsorb at the air–solution interface (
Scheme 1). Therefore, the recovery to the initial sandwich-entities is actually impossible/incomplete at the cooling back to ambient temperature (20 °C) in the samples with air–solution interfaces. The higher the heating temperature (85 °C as juxtaposed to 60 °C), and the longer the incubation time at the highest temperatures of the respective thermal cycle (30 min as juxtaposed to 4 h), the more expressed this effect (see
Figure 5a,b).
So, it might be expected that in the particular aqueous solution formulation (C(T4) = 2 × 10−4 mol/L; C(LPS) = 0.5 µg/L) at the highest investigated temperature (~80 °C) and under controlled pH = 6.2, the whole quantity of the LPS is caught in amphiphilic T4+LPS complexes. Most probably, the remaining hydrophilic tectomers are not substantially restructured in these conditions because of the PG-II-driven “click-clack” structural portions, and they remain in the bulk of the aqueous solution.
This hypothesis is still preliminary and needs further systematic investigations and should be further verified through other techniques, e.g., X-rays and neutron scattering. However, it is additionally supported by some test DLS measurements (
Figure 6).
The DLS data verify that there is no substantial change in the bulk size distribution of the structural entities before and after the heating–cooling cycle, both for the T4-only and for the T4+LPS aqueous solutions, at the temperature of 20 °C. Similarly to the case of ambient conditions [
8], the intensity is determined mainly by the size distribution of the tectomeric aggregates. The registered slight shift to larger sizes in the T4 case might be related to the more intense movement of the tectomers at higher temperatures, which enhances the possibility of the onset of some larger tectomer species formed by the initially smaller T4 entities in the cooling branch of the procedure. However, additional and more consistent studies are needed to verify such a hypothesis.