Saturated Micellar Networks: Phase Separation and Nanoemulsification Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Formation and Rheological Properties of Saturated Micellar Phases
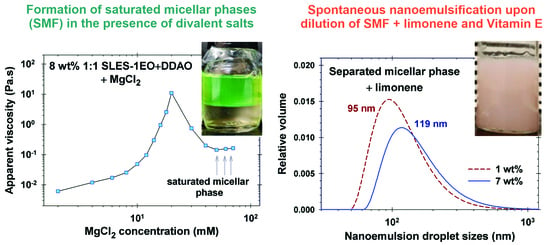
3.1. Formation of Saturated Micellar Phase
3.2. Rheology of Micellar Surfactant Solutions in the Presence of Monovalent and Divalent Salts
4. Nanoemulsification of Limonene and Vitamin E
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappelaere, E.; Cressely, R. Rheological behavior of an elongated micellar solution at low and high salt concentrations. Colloid. Polym. Sci. 1998, 276, 1050–1056. [Google Scholar] [CrossRef]
- Cappelaere, E.; Cressely, R. Influence of NaClO3 on the rheological behavior of a micellar solution of CPCl. Rheol. Acta 2000, 39, 346–353. [Google Scholar] [CrossRef]
- Nicolas-Morgantini, L. Giant micelles and shampoos. In Giant Micelles. Properties and Application; Zana, R., Kaler, E.W., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 493–514. ISBN 1420007122, 978-1420007121. [Google Scholar]
- Parker, A.; Fieber, W. Viscoelasticity of anionic wormlike micelles: Effects of ionic strength and small hydrophobic molecules. Soft Matter 2013, 9, 1203–1213. [Google Scholar] [CrossRef]
- Yavrukova, V.I.; Radulova, G.M.; Danov, K.D.; Kralchevsky, P.A.; Xu, H.; Ung, Y.W.; Petkov, J.T. Rheology of mixed solutions of sulfonated methyl esters and betaine in relation to the growth of giant micelles and shampoo applications. Adv. Colloid. Interface Sci. 2020, 275, 102062. [Google Scholar] [CrossRef]
- Mitrinova, Z.; Alexandrov, H.; Denkov, N.; Tcholakova, S. Effect of counter-ion on rheological properties of mixed surfactant solutions. Colloids Surf. A 2022, 643, 128746. [Google Scholar] [CrossRef]
- Porte, G.; Gomati, R.; El Haitamy, O.; Appell, J.; Marignan, J. Morphological transformations of the primary surfactant structures in brine-rich mixtures of ternary systems (surfactant/alcohol/brine). J. Phys. Chem. 1986, 90, 5746–5751. [Google Scholar] [CrossRef]
- Drye, T.J.; Cates, M.E. Living networks: The role of cross-links in entangled surfactant solutions. J. Chem. Phys. 1992, 96, 1367–1375. [Google Scholar] [CrossRef]
- Candau, S.J.; Khatory, A.; Lequeux, F.; Kern, F. Rheological behavior of wormlike micelles: Effect of salt content. J. Phys. IV 1993, 3, 197–209. [Google Scholar] [CrossRef]
- Khatory, A.; Kern, F.; Lequeux, F.; Appell, J.; Porte, G.; Morie, N.; Ott, A.; Urbach, W. Entanglement versus multiconnected network of wormlike micelles. Langmuir 1993, 9, 933–939. [Google Scholar] [CrossRef]
- Lequeux, F.; Candau, S.J. Dynamical properties of wormlike micelles. In Structure and Flow in Surfactant Solutions; Herb, C.A., Prud’homme, R.K., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 51–62. ISBN 0841230544, 978-0841230545. [Google Scholar]
- Cates, M.E.; Fielding, S.M. Rheology of giant micelles. Ads. Phys. 2006, 55, 799–879. [Google Scholar] [CrossRef]
- Nettesheim, F. Phase behavior of systems with wormlike micelles. In Giant Micelles. Properties and Application; Zana, R., Kaler, E.W., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 223–248. ISBN 1420007122, 978-1420007121. [Google Scholar]
- Appel, J.; Porte, G. Cloud point in ionic surfactant solutions. J. Phys. Lett. 1983, 44, L-689–L-695. [Google Scholar] [CrossRef]
- Magid, L.J. The surfactant-polyelectrolyte analogy. J. Phys. Chem. B 1998, 102, 4064–4074. [Google Scholar] [CrossRef]
- Kato, T.; Terao, T.; Seimiya, T. Intermicellar migration of surfactant molecules in entangled micellar solutions. Langmuir 1994, 10, 4468–4474. [Google Scholar] [CrossRef]
- Kato, T. Surfactant self-diffusion and networks of wormlike micelles in concentrated solutions of nonionic surfactants. Progr. Colloid. Polym. Sci. 1996, 100, 15–18. [Google Scholar] [CrossRef]
- Bernheim-Groswasser, A.; Wachtel, E.; Talmon, Y. Micellar growth, network formation and criticality in aqueous solutions of the nonionic surfactant C12E5. Langmuir 2000, 16, 4131–4140. [Google Scholar] [CrossRef]
- Nilsson, F.; Söderman, O.; Reimer, J. Phase separation and aggregate-aggregate interactions in the C9G1/C10G1 β-Alkyl Glucosides/Water System. A Phase Diagram and NMR Self-Diffusion Study. Langmuir 1998, 14, 6396–6402. [Google Scholar] [CrossRef]
- Koehler, R.D.; Raghavan, S.R.; Kaler, E.W. Microstructure and dynamics of wormlike micellar solutions formed by mixing cationic and anionic surfactants. J. Phys. Chem. B 2000, 104, 11035–11044. [Google Scholar] [CrossRef]
- Zana, R.; Kaler, E.W. (Eds.) Linear rheology of aqueous solutions of wormlike micelles. In Giant Micelles. Properties and Application; Zana, R.; Kaler, E.W. (Eds.) Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 249–287. ISBN 1420007122, 978-1420007121. [Google Scholar]
- Rogers, S.A.; Calabrese, M.A.; Wagner, N.J. Rheology of branched wormlike micelles. Curr. Opin. Colloid. Interface Sci. 2014, 19, 530–535. [Google Scholar] [CrossRef]
- Georgiev, M.T.; Aleksova, L.A.; Kralchevsky, P.A.; Danov, K.D. Phase separation of saturated micellar network and its potential application for nanoemulsification. Colloids Surf. A 2020, 607, 125487. [Google Scholar] [CrossRef]
- Patel, R.B.; Patel, M.R.; Thakore, S.D.; Patel, B.G. Nanoemulsion as a valuable nanostructure platform for pharmaceutical drug delivery. In Nano-and Microscale Drug Delivery Systems; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 321–341. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Anton, N.; Vandamme, T.F. Oral pellets loaded with nanoemulsions. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–230. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Nanoemulsion preparation, characterization, and application in the field of biomedicine. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–188. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Gorain, B.; Chatterjee, B.; Madheswaran, T.; Md, S.; Mak, K.-K.; Tambuwala, M.; Chourasia, M.K.; Kesharwani, P. Nanoemulsions as effective carriers for the treatment of lung cancer. In Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer; Kesharwani, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–247. ISBN 9780128163672. [Google Scholar]
- Szczepanowicz, K.; Hoel, H.J.; Szyk-Warszynska, L.; Bielańska, E.; Bouzga, A.M.; Gaudernack, G.; Simon, C.; Warszynski, P. Formation of biocompatible nanocapsules with emulsion core and pegylated shell by polyelectrolyte multilayer adsorption. Langmuir 2010, 26, 12592–12597. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Podgórna, K.; Szyk-Warszyńska, L.; Warszyński, P. Formation of oil filled nanocapsules with silica shells modified by sequential adsorption of polyelectrolytes. Colloids Surf. A 2014, 441, 885–889. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Bzowska, M.; Kruk, T.; Karabasz, A.; Bereta, J.; Warszynski, P. Pegylated polyelectrolyte nanoparticles containing paclitaxel as a promising candidate for drug carriers for passive targeting. Colloids Surf. B 2016, 143, 463–471. [Google Scholar] [CrossRef]
- Miller, J.H.; Quebedeaux, D.A.; Sauer, J.D. Amine oxide/alcohol ethoxylate blends: Zero-phosphate, high-performance, hard-surface cleaners. J. Am. Oil Chem. Soc. 1995, 72, 857–859. [Google Scholar] [CrossRef]
- Kolp, D.G.; Laughlin, R.G.; Krause, F.P.; Zimmerer, R.E. Interaction of dimethyldodecylamine oxide with sodium dodecylbenzenesulfonate in dilute solution. J. Phys. Chem. 1963, 67, 51–55. [Google Scholar] [CrossRef]
- Weers, J.G.; Rathman, J.F.; Scheuing, D.R. Structure/performance relationships in long chain dimethylamine oxide/sodium dodecylsulfate surfactant mixtures. Colloid. Polym. Sci. 1990, 268, 832–846. [Google Scholar] [CrossRef]
- Soontravanich, S.; Munoz, J.A.; Scamehorn, J.F.; Harwell, J.H.; Sabatini, D.A. Interaction between and anionic and an amphoteric surfactant. Part I: Monomer-micelle equilibrium. J. Surfact. Deterg. 2008, 11, 251–261. [Google Scholar] [CrossRef]
- Soontravanich, S.; Walsh, S.; Scamehorn, J.F.; Harwell, J.H.; Sabatini, D.A. Interaction between and anionic and an amphoteric surfactant. Part II: Precipitation. J. Surfact. Deterg. 2009, 12, 145–154. [Google Scholar] [CrossRef]
- Kakitani, M.; Imae, T.; Furusaka, M. Investigation of mixed micelles of dodecyldimethylamine oxide and sodium dodecyl sulfate by SANS: Shape, size, charge, and interaction. J. Phys. Chem. 1995, 99, 16018–16023. [Google Scholar] [CrossRef]
- Varade, D.; Joshi, T.; Aswal, V.K.; Goyal, P.S.; Hassan, P.A.; Bahadur, P. Micellar behavior of mixtures of sodium dodecyl sulfate and dodecyldimethylamine oxide in aqueous solutions. Colloids Surf. A 2005, 259, 103–109. [Google Scholar] [CrossRef]
- Goloub, T.P.; Pugh, R.J.; Zhmud, B.V. Micellar interactions in nonionic/ionic mixed surfactant systems. J. Colloid. Interf. Sci. 2000, 229, 72–81. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Murch, B.; Pukinsky, I.B.; Churjusova, T.G.; Alexeeva, M.V.; Vlasov, A.Y.; Mokrushina, L.V. Phase boundaries for mixed aqueous micellar solutions of dimethyldodecylamine oxide and sodium or magnesium dodecyl sulfate with regard to chemical processes in the systems. Langmuir 2002, 18, 3446–3453. [Google Scholar] [CrossRef]
- Khodaparast, S.; Sharratt, W.N.; Tyagi, G.; Dalgliesh, R.M.; Robles, E.S.J.; Cabral, J.T. Pure and mixed aqueous micellar solutions of sodium dodecyl sulfate (SDS) and dimethyldodecyl amine oxide (DDAO): Role of temperature and composition. J. Colloid. Interf. Sci. 2021, 582, 1116–1127. [Google Scholar] [CrossRef]
- Tyagi, G.; Seddon, D.; Khodaparast, S.; Sharratt, W.N.; Roble, E.S.J.; Cabral, J.T. Tensiometry and FTIR study of the synergy in mixed SDS:DDAO surfactant solution in varying pH. Colloids Surf. A 2021, 618, 126414. [Google Scholar] [CrossRef]
- Safonova, E.A.; Alexeeva, M.V.; Smirnova, N.A. The effect of acidity on micellization in dodecyldimethylamine oxide-sodium dodecyl sulfate aqueous mixtures. Colloid. J. 2009, 71, 717–724. [Google Scholar] [CrossRef]
- Safonova, E.A.; Alekseeva, M.V.; Filippov, S.K.; Durrschmidt, T.; Mokrushina, L.V.; Hoffmann, H.; Smirnova, N.A. The structure and rheology of mixed micellar solutions of sodium dodecyl sulfate and dodecyldimethylamine oxide. Russ. J. Phys. Chem. 2006, 80, 915–921. [Google Scholar] [CrossRef]
- Summerton, E.; Hollamby, M.J.; Le Duff, C.S.; Thompson, E.S.; Snow, T.; Smith, A.J.; Jones, C.; Bettiol, J.; Bakalis, S.; Britton, M.M. Nuclear magnetic resonance and small-angle X-ray scattering studies of mixed sodium dodecyl sulfate and N,N-dimethyldodecylamine N-oxide aqueous systems performed at low temperatures. J. Colloid. Interf. Sci. 2019, 535, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Hoffmann, H.; Horbaschek, K. A novel cationic/anionic surfactant system from a zwitterionic alkyldimethylamine oxide and dihydroperfluorooctanoic acid. Langmuir 2001, 17, 4151–4160. [Google Scholar] [CrossRef]
- Hoffmann, H.; Thunig, C.; Valiente, M. The different phases and their macroscopic properties in ternary surfactant systems of alkyldimethylamine oxides, intermediate chain n-alcohols and water. Colloids Surf. 1992, 67, 223–237. [Google Scholar] [CrossRef]
- Blagojević, S.M.; Pejić, N.D.; Blagojević, S.N. Synergism and physicochemical properties of anionic/amphoteric surfactant mixtures with nonionic surfactant of amine oxide type. Russ. J. Phys. Chem. 2017, 91, 2690–2695. [Google Scholar] [CrossRef]
- Lin, Z. Branched worm-like micelles and their networks. Langmuir 1996, 12, 1729–1737. [Google Scholar] [CrossRef]
- Birnie, C.R.; Malamud, D.; Schnaare, R.L. Antimicrobial Evaluation of N-Alkyl Betaines and N-Alkyl-N,N-Dimethylamine Oxides with Variations in Chain Length. Antimicrob. Agents Chemother. 2000, 44, 2514–2517. [Google Scholar] [CrossRef]
- Singh, S.K.; Bajpai, M.; Tyagi, V.K. Amine oxides: A review. J. Oleo Sci. 2006, 55, 99–119. [Google Scholar] [CrossRef]
- Garcia, M.T.; Campos, E.; Ribosa, I. Biodegradability and ecotoxicity of amine oxide-based surfactants. Chemosphere 2007, 69, 1574–1578. [Google Scholar] [CrossRef]
- Stancheva, T.S.; Georgiev, M.T.; Radulova, G.M.; Danov, K.D.; Marinova, K.G. Rheology of saturated micellar networks: Wormlike micellar solutions vs. bicontinuous micellar phases. Colloids Surf. A 2022, 652, 129927. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Niederholtmeyer, H.; Podolsky, K.A.; Bhattacharya, R.; Song, J.-J.; Brea, R.J.; Tsai, C.-H.; Sinha, S.K.; Devaraj, N.K. Lipid sponge droplets as programmable synthetic organelles. Proc. Natl. Acad. Sci. USA 2020, 117, 18206–18215. [Google Scholar] [CrossRef] [PubMed]
- Tartaro, G.; Gentile, L.; Palazzo, G. Characteristic length and curvature of AOT/brine/squalene “sponge” L3 phases. J. Colloid. Interface Sci. Open 2023, 9, 100077. [Google Scholar] [CrossRef]
- Gilbert, J.; Christensen, S.; Nylander, T.; Bülow, L. Encapsulation of sugar beet phytoglobin BvPgb 1.2 and myoglobin in a lipid sponge phase system. Front. Soft. Matter. 2023, 3, 1201561. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Tiberg, F. Self-assembled lipid superstructures: Beyond vesicles and liposomes. Nano Lett. 2005, 8, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Valldeperas, M.; Wisniewska, M.; Ram-On, M.; Kesselman, E.; Danino, D.; Nylander, T.; Barauskas, J. Sponge phases and nanoparticle dispersions in aqueous mixtures of mono- and diglycerides. Langmuir 2016, 32, 8650–8659. [Google Scholar] [CrossRef] [PubMed]
- Christov, N.C.; Denkov, N.D.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P.; Lips, A. Synergistic sphere-to-rod micelle transition in mixed solutions of sodium dodecyl sulfate and cocoamidopropyl betaine. Langmuir 2004, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; de Medina, P.; Caze-Subra, S.; Bystricky, K.; Balaguer, P.; Poirot, M.; Silvente-Poirot, S. Molecular and biochemical analysis of the estrogenic and proliferative properties of Vitamin E compounds. Front. Oncol. 2016, 5, 287. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavova, T.G.; Radulova, G.M.; Danov, K.D. Saturated Micellar Networks: Phase Separation and Nanoemulsification Capacity. Colloids Interfaces 2024, 8, 11. https://doi.org/10.3390/colloids8010011
Slavova TG, Radulova GM, Danov KD. Saturated Micellar Networks: Phase Separation and Nanoemulsification Capacity. Colloids and Interfaces. 2024; 8(1):11. https://doi.org/10.3390/colloids8010011
Chicago/Turabian StyleSlavova, Tatiana G., Gergana M. Radulova, and Krassimir D. Danov. 2024. "Saturated Micellar Networks: Phase Separation and Nanoemulsification Capacity" Colloids and Interfaces 8, no. 1: 11. https://doi.org/10.3390/colloids8010011
APA StyleSlavova, T. G., Radulova, G. M., & Danov, K. D. (2024). Saturated Micellar Networks: Phase Separation and Nanoemulsification Capacity. Colloids and Interfaces, 8(1), 11. https://doi.org/10.3390/colloids8010011