Twinning in Zr-Based Metal-Organic Framework Crystals
Abstract
:1. Introduction
- (a)
- A crystal consisting of two or more domains related by seemingly arbitrary rotation matrices (although not technically twinning), is frequently encountered when investigating MOF crystals. It occurs when two randomly oriented crystals in close proximity grow into each other and forms an interface, and is frequently observed in static syntheses where crystal growth mainly occurs on the bottom of the synthesis vessel. In these cases, automatic indexing fails to provide a meaningful unit cell, but the relationship can usually be determined by manual inspection and sorting of the reflections in reciprocal space.
- (b)
- In certain cases, automatic indexing found a hexagonal supercell due to partial overlap between the reflections from the twin domains. The twin law was found to be the so-called “spinel law”, 2[111] (where the two twin domains are related by a two-fold rotation about the body diagonal of a cubic unit cell), which is a case of twinning by reticular merohedry [10]. This twinning mode was observed in UiO-67, UiO-67-Me2 (1, discussed herein) and Zr-stilbene dicarboxylate. In all of these cases, the crystals displayed a specific morphology, resembling intergrown octahedra with two shared (1 1 1) faces (Figure 1).
- (c)
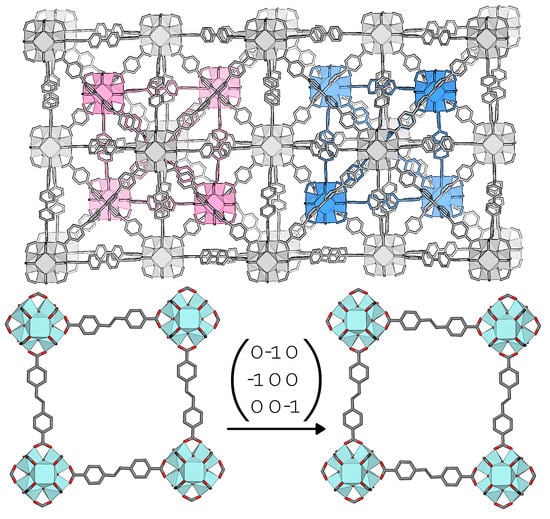
- In certain cases, twinning by syngonic merohedry was observed as a consequence of intrinsic features of the MOF. The examples presented herein are obtained from MOFs featuring partial lattice interpenetration (3) and a phase transitions from dynamic to static orientation disorder upon cooling of the sample (2).
2. Materials and Methods
2.1. General Materials and Synthesis Methods
2.2. X-ray Crystallography
3. Results and Discussion
3.1. UiO-67-Me2
3.2. Zr-stilbene Dicarboxylate
3.3. Interpenetrated UiO-69-Me2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [Green Version]
- Öhrström, L. Let’s Talk about MOFs—Topology and Terminology of Metal-Organic Frameworks and Why We Need Them. Crystals 2015, 5, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Gandara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal-Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal-organic frameworks: From nano to single crystals. Chem.-Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Gandara, F.; Bennett, T.D. Crystallography of metal-organic frameworks. IUCrJ 2014, 1, 563–570. [Google Scholar] [CrossRef]
- Parsons, S. Introduction to twinning. Acta Crystallogr. Sect. D 2003, 59, 1995–2003. [Google Scholar] [CrossRef] [Green Version]
- Klapper, H.; Hahn, T. The application of eigensymmetries of face forms to X-ray diffraction intensities of crystals twinned by ‘reticular merohedry’. Acta Crystallogr. Sect. A 2012, 68, 82–109. [Google Scholar] [CrossRef]
- Hylland, K.T.; Øien-Ødegaard, S.; Lillerud, K.P.; Tilset, M. Efficient, Scalable Syntheses of Linker Molecules for Metal-Organic Frameworks. Synlett 2015, 26, 1480–1485. [Google Scholar] [CrossRef]
- Ursby, T.; Unge, J.; Appio, R.; Logan, D.T.; Fredslund, F.; Svensson, C.; Larsson, K.; Labrador, A.; Thunnissen, M.M.G.M. The macromolecular crystallography beamline I911-3 at the MAX IV laboratory. J. Synchrotron Radiat. 2013, 20, 648–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruker APEX3, SAINT, SADABS, XPREP, 2019.1-1; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Flack, H. Methods of space-group determination—A supplement dealing with twinned crystals and metric specialization. Acta Crystallogr. Sect. C 2015, 71, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Øien-Ødegaard, S.; Bouchevreau, B.; Hylland, K.; Wu, L.; Blom, R.; Grande, C.; Olsbye, U.; Tilset, M.; Lillerud, K.P. UiO-67-type Metal–Organic Frameworks with Enhanced Water Stability and Methane Adsorption Capacity. Inorg. Chem. 2016, 55, 1986–1991. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Castillo-Martínez, E.; Wu, Y.; Lee, J.; Forse, A.C.; Firth, F.C.N.; Moghadam, P.Z.; Fairen-Jimenez, D.; Gaultois, M.W.; Hill, J.A.; et al. Metal–Organic Nanosheets Formed via Defect-Mediated Transformation of a Hafnium Metal–Organic Framework. J. Am. Chem. Soc. 2017, 139, 5397–5404. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Marshall, R.J.; Hobday, C.L.; Murphie, C.F.; Griffin, S.L.; Morrison, C.A.; Moggach, S.A.; Forgan, R.S. Amino acids as highly efficient modulators for single crystals of zirconium and hafnium metal-organic frameworks. J. Mater. Chem. A 2016, 4, 6955–6963. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Yuan, S.; Qin, J.-S.; Wang, Y.; Kirchon, A.; Qiu, D.; Cheng, L.; Madrahimov, S.T.; Zhou, H.-C. Lattice Expansion and Contraction in Metal-Organic Frameworks by Sequential Linker Reinstallation. Matter 2019, 1, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Lippke, J.; Brosent, B.; von Zons, T.; Virmani, E.; Lilienthal, S.; Preuße, T.; Hülsmann, M.; Schneider, A.M.; Wuttke, S.; Behrens, P.; et al. Expanding the Group of Porous Interpenetrated Zr-Organic Frameworks (PIZOFs) with Linkers of Different Lengths. Inorg. Chem. 2017, 56, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Schaate, A.; Roy, P.; Preuße, T.; Lohmeier, S.J.; Godt, A.; Behrens, P. Porous Interpenetrated Zirconium–Organic Frameworks (PIZOFs): A Chemically Versatile Family of Metal–Organic Frameworks. Chem.-Eur. J. 2011, 17, 9320–9325. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Liu, L.; Tapperwijn, S.J.; Perl, D.; Coudert, F.-X.; Van Cleuvenbergen, S.; Verbiest, T.; van der Veen, M.A.; Telfer, S.G. Controlled partial interpenetration in metal–organic frameworks. Nat. Chem. 2016, 8, 250–257. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |







| Crystal Data | UiO-67-Me2 (1) | Zr-Stilbene dc (2) | UiO-69-Me2 (3) |
|---|---|---|---|
| Chemical formula | C384O128Zr24·32(O) | C342.25H211.09O128Zr24 | C648H408O128Zr24· 0.66(C648H408O128Zr24)· 16(O) |
| Mr | 2340.28 | 8584.82 | 20,698.65 |
| Crystal system, space group | Cubic, Fmm | Cubic, Pn | Cubic, F m |
| Temperature (K) | 100 | 100 | 100 |
| a (Å) | 26.8903 (12) | 30.0322 (6) | 38.995 (2) |
| V (Å3) | 19,444 (3) | 27,087.0 (16) | 59,298 (10) |
| Radiation type | Mo Kα | Synchrotron, λ = 0.760 Å | Synchrotron, λ = 0.3112 Å |
| µ (mm−1) | 0.35 | 0.25 | 0.17 |
| Crystal size (mm) | 0.06 × 0.06 × 0.02 | 0.2 × 0.2 × 0.2 | 0.14 × 0.14 × 0.14 |
| Data collection | |||
| Diffractometer | Bruker D8 Venture, CMOS detector | MD2 microdiffractometer with MK3 mini-kappa | ESRF ID11 |
| Absorption correction | Multi-scan | Multi-scan | Multi-scan |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4388, 4388, 4155 | 199,694, 11,196, 10,761 | 180,671, 19,944, 17,103 |
| Rint | 0.036 | 0.04 | 0.033 |
| (sin θ/λ)max (Å−1) | 0.649 | 0.623 | 0.961 |
| Refinement | |||
| R[F2 > 2σ(F2)], wR(F2), S | 0.048, 0.174, 1.13 | 0.029, 0.090, 1.10 | 0.047, 0.154, 1.08 |
| No. of reflections | 4388 | 11,196 | 19,944 |
| No. of parameters | 59 | 216 | 226 |
| No. of restraints | 0 | 18 | 304 |
| Δρmax, Δρmin (e Å−3) | 1.03, −1.05 | 0.91, −0.46 | 2.17, −2.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Øien-Ødegaard, S.; Lillerud, K.P. Twinning in Zr-Based Metal-Organic Framework Crystals. Chemistry 2020, 2, 777-786. https://doi.org/10.3390/chemistry2030050
Øien-Ødegaard S, Lillerud KP. Twinning in Zr-Based Metal-Organic Framework Crystals. Chemistry. 2020; 2(3):777-786. https://doi.org/10.3390/chemistry2030050
Chicago/Turabian StyleØien-Ødegaard, Sigurd, and Karl Petter Lillerud. 2020. "Twinning in Zr-Based Metal-Organic Framework Crystals" Chemistry 2, no. 3: 777-786. https://doi.org/10.3390/chemistry2030050
APA StyleØien-Ødegaard, S., & Lillerud, K. P. (2020). Twinning in Zr-Based Metal-Organic Framework Crystals. Chemistry, 2(3), 777-786. https://doi.org/10.3390/chemistry2030050