Erdmann’s Anion—An Inexpensive and Useful Species for the Crystallization of Illicit Drugs after Street Confiscations †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses and Crystallization
2.1.1. Syntheses of (1) and (2)
2.1.2. Preparation of the Drug Crystals
2.2. Crystallographic Studies
3. Results
3.1. Structures (1) and (2)
3.1.1. The Potassium Salt of Erdmann’s Anion (1)
3.1.2. Structure of the Ammonium Salt of Erdmann’s Anion (2)
3.2. Structures of the Erdmann’s Complexes with Various Street Drugs (3), (4), (5)
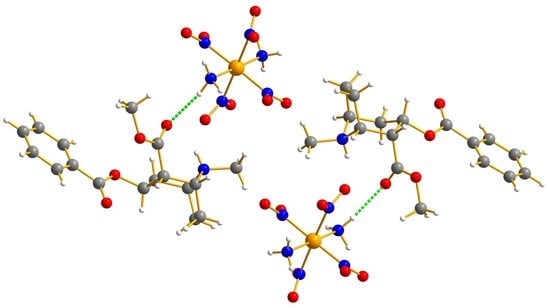
3.2.1. Erdmann’s Salt of Cocaine, C17H28CoN7O12 (3)
3.2.2. The Methamphetamine–Erdmann’s Complex (4)
3.2.3. The Methylone–Erdmann’s Complex (5)
3.3. Packing Considerations in the Three Drug Complexes
3.3.1. Overlay Diagrams of the Drug Fragments for the Complexes with Cocaine (3) and with Methamphetamine (4)
3.3.2. Racemic Mimics
- a. The Case of Cocaine (3)

- b. The Case of Methamphetamine (4)
3.3.3. π–π Contacts
- a. Cocaine Cation with Erdmann’s Anion (3)
- b. Methamphetamine Cation with Erdmann’s Anion
- c. Methylone Cation with Erdmann’s Anion (5)
4. Summary of Experimental Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dymock, W.; Warden, C.H.J.; Hooper, D. Pharmacographia Indica: A History of the Principal Drugs of the Vegetable Origin; Kegan, Paul, Trench, Trubner & Co., Ld.: London, UK, 1893; pp. 261–265. [Google Scholar]
- Shintani, H.; Sato, S.; Saito, Y. The crystal structure of (−)589 dinitrobis(ethylenediamine)-cobalt(III)(-)589-dinitrooxolatodiamm inecobaltate(III) monohydrate. Acta Cryst. 1976, B32, 1184–1188. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, J.; Lightfoot, M.P.; Ward, S.C. CSD = CCDC = The Cambridge Structural Database. (v4.0.0) of 2019. Acta Cryst. 2016, B72m, 171–179. [Google Scholar] [CrossRef]
- Wood, M.R.; Thompson, H.W.; Brettell, T.A.; Lalancette, R.A. The hydrated and anhydrous gold(III) tetrachloride salts of L-ecgonine, and important forensic toxicology marker for cocaine. Acta Cryst. 2010, C66, m4–m8. [Google Scholar] [CrossRef]
- Wood, M.R.; Lalancette, R.A.; Bernal, I. Crystallographic investigations of select cathinones: Emerging illicit street drugs known as ‘bath salts’. Acta Cryst. 2015, C71, 32–38. [Google Scholar] [CrossRef]
- Erdmann, O.L. Ueber einige salpetrigsaure Nickel- und Kobalt- verbindungen. J. Prakt. Chem. 1866, 97, 385–413. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, S.M. Preparation of cobalt-ammonia salts. Zeit. Anorg. Chem. 1898, 17, 455–479. [Google Scholar]
- Komiyama, Y. The crystal structure of potassium tetranitro-diammine-cobaltiate(III), K[Co(NH3)2(NO2)4]. Bull. Chem. Soc. Jpn. 1956, 29, 300–304. [Google Scholar] [CrossRef] [Green Version]
- Bruker. SADABS, SAINT and SMART; Bruker AXS, Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXT. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Flack, H.D. Physical and spectrometric analysis: Absolute configuration determination by X-ray crystallography. Compr. Chirality 2012, 8, 648–656. [Google Scholar]
- Bernal, I. Proceedings of the ACA Annual Meeting, Montreal, QC, Canada; 1995. (Abstract 4a.1.e).
- Bernal, I.; Watkins, S.F. A list of organoetallic kryptoracemates. Acta Cryst. 2015, C71, 216–221. [Google Scholar]
- Clevers, S.; Coquerel, G. Kryptoracemic compound hunting and frequency in the Cambridge Structural Database. Cryst. Eng. Comm. 2020, 22, 7407–7419. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. DIAMOND, Version 8.5.10.; GbR, Kreuzherrenstr.: Bonn, Germany, 2019. [Google Scholar]
- Furberg, S.; Hassel, O. Remarks on the crystal structure of asymmetric molecules. The phenylglyceric acids. Acta Chem. Scand. 1950, 1020–1023. [Google Scholar] [CrossRef]
- Schouwstra, Y. Crystal and molecular structures of DL-Methylsuccinic acid, I. A modification obtained by sublimation at 70 °C in vacuo. Acta Cryst. 1973, B29, 1–4. [Google Scholar]
- Schouwstra, Y. Crystal and molecular structures of DL-methylsuccinic acid, II. Two modifications obtained by slow evaporation of aqueous solutions. Acta Cryst. 1973, B29, 1636–1640. [Google Scholar] [CrossRef]
- Mostad, A.; Rømming, C.; Tressum, L. The crystal and molecular structure of (2-Hydroxyphenyl)alanine (oTyrosine). Acta Chem. Scand. 1975, B29, 171–176. [Google Scholar] [CrossRef]
- Herbstein, F.H. Crystalline Molecular Complexes and Compounds; Oxford University Press: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Janiak, C. A critical account of π-π stacking in metal complexes with aromatic nitrogen- containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Scientific Working Group for the Analysis of Seized Drugs. SWGDrug Guidelines. Available online: http://www.swgdrug.org (accessed on 30 July 2020).











| Crystal Data | ||
| (1) = Potassium salt | (2) = Ammonium salt | |
| Chemical formula | CoH6KN6O8 | CoH10N7O8 |
| Mr | 316.14 | 295.08 |
| Crystal system, space group | Orthorhombic, P212121 | Orthorhombic, P212121 |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 6.6678(4), 11.1459(7), 12.7205(9) | 6.6760(2), 11.3965(4), 12.7830(4) |
| α, β, γ (°) | 90., 90., 90. | 90., 90., 90. |
| V (Å3) | 945.37(11) | 972.57(5) |
| Z, Z’ | 4, 1 | 4, 1 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 18.733 | 14.415 |
| Crystal size (mm) | 0.186 × 0.230 × 0.351 | 0.100 × 0.131 × 0.152 |
| Data Collection | ||
| Diffractometer | Bruker APEX2 | Bruker APEX2 |
| Absorption correction | numerical | numerical |
| Tmin, Tmax | 0.053, 0.175 | 0.210, 0.409 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 7865, 1473, 1459 | 8624, 1632, 1569 |
| Rint | 0.034 | 0.027 |
| (sin θ/λ)max (Å−1) | 0.610 | 0.618 |
| Refinement | ||
| R[F > 2σ(F)], wR(F), S | 0.019, 0.047, 1.06 | 0.021, 0.048, 1.02 |
| No. of refl., params., restraints | 1473, 164, 0 | 1632, 175, 4 |
| H−atom treatment | refxyz | refxyz |
| Flack parameter | −0.011(5) | 0.030(4) |
| CCDC number | 2047594 | 2047595 |
| Crystal Data | |||
| (3) = cocaine salt | (4) = methamphetamine salt | (5) = methylone salt | |
| Chemical formula | C17H28CoN7O12 | C10H22CoN7O8 | C22H34CoN8O14 |
| Mr | 581.39 | 427.27 | 693.50 |
| Crystal system, space group | Triclinic, P1 | Monoclinic, P21 | Triclinic, P−1 |
| Temperature (K) | 100 | 100 | 100 |
| a, b, c (Å) | 6.2403(3), 11.0319(4), 18.9421(7) | 6.3873(2), 13.0182(3), 21.6772(5) | 7.0437(4), 10.3155(7), 10.7697(7) |
| α, β, γ (°) | 106.450(2), 93.831(2), 92.655(2) | 90., 94.6700(17), 90. | 90.712(5), 106.330(4), 107.985(4) |
| V (Å3) | 1244.94(9) | 1796.50(8) | 710.02(8) |
| Z, Z’ | 2, 2 | 4, 2 | 1, 1 |
| Radiation type | Cu Kα | Cu Kα | Cu Kα |
| μ (mm−1) | 6.07 | 8.01 | 5.50 |
| Crystal size (mm) | 0.04 × 0.09 × 0.23 | 0.06 × 0.10 × 0.50 | 0.11 × 0.18 × 0.65 |
| Data Collection | |||
| Diffractometer | Bruker APEX2 | Bruker APEX2 | Bruker APEX2 |
| Absorption correction | numerical | numerical | numerical |
| Tmin, Tmax | 0.347, 0.772 | 0.440, 0.619 | 0.220, 0.682 |
| No. of measured, independent, and observed [I > 2σ(I)] refl. | 9894, 5432, 4513 | 15772, 5875, 4066 | 6733, 1302, 1100 |
| Rint | 0.030 | 0.073 | 0.029 |
| (sin θ/λ)max (Å−1) | 0.607 | 0.610 | 0.497 |
| Refinement | |||
| R[F > 2σ(F)], wR(F), S | 0.038, 0.087, 0.95 | 0.057, 0.073, 0.90 | 0.079, 0.170, 1.06 |
| No. of refl., params., restraints | 5432, 675, 3 | 5875, 471, 1 | 1302, 209, 24 |
| H−atom treatment | mixed | mixed | constr |
| Flack parameter | −0.003(4) | 0.024(6) | − |
| Δρmax, Δρmin (e Å−3) | 0.43, −0.39 | 0.65, −0.48 | 0.77, −0.43 |
| CCDC number | 2042087 | 2042090 | 2042091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, M.R.; Mikhael, S.; Bernal, I.; Lalancette, R.A. Erdmann’s Anion—An Inexpensive and Useful Species for the Crystallization of Illicit Drugs after Street Confiscations. Chemistry 2021, 3, 598-611. https://doi.org/10.3390/chemistry3020042
Wood MR, Mikhael S, Bernal I, Lalancette RA. Erdmann’s Anion—An Inexpensive and Useful Species for the Crystallization of Illicit Drugs after Street Confiscations. Chemistry. 2021; 3(2):598-611. https://doi.org/10.3390/chemistry3020042
Chicago/Turabian StyleWood, Matthew R., Sandra Mikhael, Ivan Bernal, and Roger A. Lalancette. 2021. "Erdmann’s Anion—An Inexpensive and Useful Species for the Crystallization of Illicit Drugs after Street Confiscations" Chemistry 3, no. 2: 598-611. https://doi.org/10.3390/chemistry3020042
APA StyleWood, M. R., Mikhael, S., Bernal, I., & Lalancette, R. A. (2021). Erdmann’s Anion—An Inexpensive and Useful Species for the Crystallization of Illicit Drugs after Street Confiscations. Chemistry, 3(2), 598-611. https://doi.org/10.3390/chemistry3020042