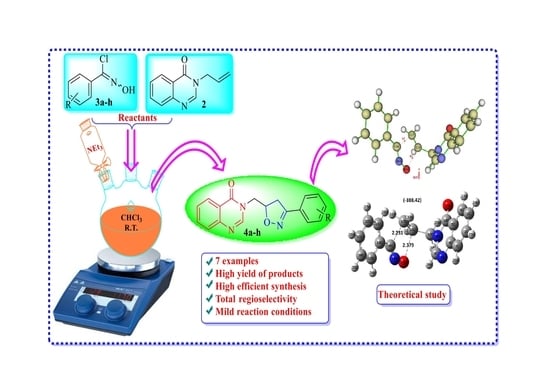
Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Instruments
2.2. Procedure for the Synthesis of Compounds (4a–h)
- 3-((3-(4-bromophenyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4a):
- 3-((3-(4-chlorophenyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4b):
- 3-((3-(4-methoxyphenyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4c):
- 3-((3-(4-nitrophenyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4d):
- 3-((3-(p-tolyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4e):
- 3-((3-phenyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4f):
- 3-((3-(2-chlorophenyl)-4,5-dihydroisoxazol-5-yl)methyl)quinazoline-4(3H)-one (4g):
2.3. Computational Details
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. DFT Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Azimi, F.; Azizian, H.; Najafi, M.; Hassanzadeh, F.; Sadeghi-aliabadi, H.; Ghasemi, J.B.; Ali Faramarzi, M.; Mojtabavi, S.; Larijani, B.; Saghaei, L.; et al. Design and Synthesis of Novel Quinazolinone-Pyrazole Derivatives as Potential α-Glucosidase Inhibitors: Structure-Activity Relationship, Molecular Modeling and Kinetic Study. Bioorganic Chem. 2021, 114, 105127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, Y.S.; Jeon, S.; Lee, S.I.; Noh, S.; Cho, J.E.; Jang, M.S.; Kim, S.; Song, J.H.; Kim, H.R.; et al. Design, Synthesis and Biological Evaluation of 2-Aminoquinazolin-4(3H)-One Derivatives as Potential SARS-CoV-2 and MERS-CoV Treatments. Bioorganic Med. Chem. Lett. 2021, 39, 127885. [Google Scholar] [CrossRef] [PubMed]
- Rishikesan, R.; Karuvalam, R.P.; Muthipeedika, N.J.; Sajith, A.M.; Eeda, K.R.; Pakkath, R.; Haridas, K.R.; Bhaskar, V.; Narasimhamurthy, K.H.; Muralidharan, A. Synthesis of Some Novel Piperidine Fused 5-Thioxo-1H-1,2,4-Triazoles as Potential Antimicrobial and Antitubercular Agents. J. Chem. Sci. 2021, 133, 3. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Chahal, M. Synthesis and Antibacterial Activity of Benzothiazole and Benzoxazole-Appended Substituted 1,2,3-Triazoles. J. Chem. Sci. 2020, 132, 142. [Google Scholar] [CrossRef]
- Jao, C.W.; Lin, W.C.; Wu, Y.T.; Wu, P.L. Isolation, Structure Elucidation, and Synthesis of Cytotoxic Tryptanthrin Analogues from Phaius Mishmensis. J. Nat. Prod. 2008, 71, 1275–1279. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent Advancements in the Development of Heterocyclic Anti-Inflammatory Agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef]
- Ashok, D.; Reddy, M.R.; Dharavath, R.; Nagaraju, N.; Ramakrishna, K.; Gundu, S.; Sarasija, M. One-Pot Three-Component Condensation for the Synthesis of 2,4,6-Triarylpyridines and Evaluation of Their Antimicrobial Activity. J. Chem. Sci. 2021, 133, 22. [Google Scholar] [CrossRef]
- Saeedi, M.; Mohammadi-Khanaposhtani, M.; Pourrabia, P.; Razzaghi, N.; Ghadimi, R.; Imanparast, S.; Faramarzi, M.A.; Bandarian, F.; Esfahani, E.N.; Safavi, M.; et al. Design and Synthesis of Novel Quinazolinone-1,2,3-Triazole Hybrids as New Anti-Diabetic Agents: In Vitro α-Glucosidase Inhibition, Kinetic, and Docking Study. Bioorganic Chem. 2019, 83, 161–169. [Google Scholar] [CrossRef] [PubMed]
- El Abbouchi, A.; El Brahmi, N.; Hiebel, M.A.; Bignon, J.; Guillaumet, G.; Suzenet, F.; El Kazzouli, S. Synthesis and Evaluation of a Novel Class of Ethacrynic Acid Derivatives Containing Triazoles as Potent Anticancer Agents. Bioorganic Chem. 2021, 115, 105293. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F.A. Recent Developments in Synthetic Chemistry and Biological Activities of Pyrazole Derivatives. J. Chem. Sci. 2019, 131, 70. [Google Scholar] [CrossRef] [Green Version]
- Kumar Pandey, S.; Yadava, U.; Upadhyay, A.; Sharma, M.L. Synthesis, Biological Evaluation and Molecular Docking Studies of Novel Quinazolinones as Antitubercular and Antimicrobial Agents. Bioorganic Chem. 2021, 108, 104611. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.W.; Yin, X.D.; Li, H.; Ma, K.Y.; Zhang, Z.J.; Zhou, R.; Wang, Y.L.; Hu, G.F.; Liu, Y.Q. Design, Synthesis, and Structure-Activity Relationship of Quinazolinone Derivatives as Potential Fungicides. J. Agric. Food Chem. 2021, 69, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone Derivatives: Promising Antibacterial Drug Leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Soliman, A.M.; Karam, H.M.; Mekkawy, M.H.; Ghorab, M.M. Antioxidant Activity of Novel Quinazolinones Bearing Sulfonamide: Potential Radiomodulatory Effects on Liver Tissues via NF-ΚB/PON1 Pathway. Eur. J. Med. Chem. 2020, 197, 112333. [Google Scholar] [CrossRef]
- Ugale, V.G.; Bari, S.B.; Khadse, S.C.; Reddy, P.N.; Bonde, C.G.; Chaudhari, P.J. Exploring Quinazolinones as Anticonvulsants by Molecular Fragmentation Approach: Structural Optimization, Synthesis and Pharmacological Evaluation Studies. ChemistrySelect 2020, 5, 2902–2912. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Chandrashekar, G.; Smith, E.; Liu, X.; Zhang, Y. Synthesis and Evaluation of 4-Quinazolinone Compounds as Potential Antimalarial Agents. Eur. J. Med. Chem. 2010, 45, 3864–3869. [Google Scholar] [CrossRef]
- Elshahawi, M.M.; EL-Ziaty, A.K.; Morsy, J.M.; Aly, A.F. Synthesis and Insecticidal Efficacy of Novel Bis Quinazolinone Derivatives. J. Heterocycl. Chem. 2016, 53, 1443–1448. [Google Scholar] [CrossRef]
- Hu, Y.G.; Yang, S.J.; Ding, M.W. Synthesis and Fungicidal Activities of 2-Benzothiazolylthio-Substituted 4H-Imidazol-4-Ones and 4(3H)-Quinazolinones. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 1933–1939. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An Overview of Quinazolines: Pharmacological Significance and Recent Developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent Advances in the Pharmacological Diversification of Quinazoline/Quinazolinone Hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Jahng, Y. Progress in the Studies on Tryptanthrin, an Alkaloid of History. Arch. Pharm. Res. 2013, 36, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic Approaches, Functionalization and Therapeutic Potential of Quinazoline and Quinazolinone Skeletons: The Advances Continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, U.A. Recent Developments in the Chemistry of Quinazolinone Alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.L.; das Neves, G.M.; Porto Kagami, L.; Eifler-Lima, V.L.; Merlo, A.A. Discovery, Development, Chemical Diversity and Design of Isoxazoline-Based Insecticides. Bioorganic Med. Chem. 2021, 30, 115934. [Google Scholar] [CrossRef]
- Asahi, M.; Kobayashi, M.; Kagami, T.; Nakahira, K.; Furukawa, Y.; Ozoe, Y. Fluxametamide: A Novel Isoxazoline Insecticide That Acts via Distinctive Antagonism of Insect Ligand-Gated Chloride Channels. Pestic. Biochem. Physiol. 2018, 151, 67–72. [Google Scholar] [CrossRef]
- Romero-Núñez, C.; Bautista-Gómez, L.G.; Sheinberg, G.; Martín-Cordero, A.; Flores-Ortega, A.; Heredia-Cárdenas, R. Efficacy of Afoxolaner plus Milbemycin Oxime and Afoxolaner Alone as Treatment for Sarcoptic Mange in Naturally Infested Dogs. Can. J. Vet. Res. 2020, 84, 212–216. [Google Scholar]
- Kreuzer, J.; Bach, N.C.; Forler, D.; Sieber, S.A. Target Discovery of Acivicin in Cancer Cells Elucidates Its Mechanism of Growth Inhibition. Chem. Sci. 2015, 6, 237–245. [Google Scholar] [CrossRef]
- Filali, I.; Bouajila, J.; Znati, M.; Bousejra-El Garah, F.; Ben Jannet, H. Synthesis of New Isoxazoline Derivatives from Harmine and Evaluation of Their Anti-Alzheimer, Anti-Cancer and Anti-Inflammatory Activities. J. Enzym. Inhib. Med. Chem. 2015, 30, 371–376. [Google Scholar] [CrossRef]
- Alshamari, A.; Al-Qudah, M.; Hamadeh, F.; Al-Momani, L.; Abu-Orabi, S. Synthesis, Antimicrobial and Antioxidant Activities of 2-Isoxazoline Derivatives. Molecules 2020, 25, 4271. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Nefzi, A. Solid Phase Synthesis of Isoxazole and Isoxazoline-Carboxamides via [2+3]-Dipolar Cycloaddition Using Resin-Bound Alkynes or Alkenes. Tetrahedron Lett. 2012, 53, 2096–2099. [Google Scholar] [CrossRef]
- Kamal, A.; Bharathi, E.V.; Reddy, J.S.; Ramaiah, M.J.; Dastagiri, D.; Reddy, M.K.; Viswanath, A.; Reddy, T.L.; Shaik, T.B.; Pushpavalli, S.N.C.V.L.; et al. Synthesis and Biological Evaluation of 3,5-Diaryl Isoxazoline/Isoxazole Linked 2,3-Dihydroquinazolinone Hybrids as Anticancer Agents. Eur. J. Med. Chem. 2011, 46, 691–703. [Google Scholar] [CrossRef]
- Chalkha, M.; Bakhouch, M.; Akhazzane, M.; Bourass, M.; Nicolas, Y.; Al Houari, G.; El Yazidi, M. Design, Synthesis and Characterization of Functionalized Pyrazole Derivatives Bearing Amide and Sulfonamide Moieties from Aza-Aurones. J. Chem. Sci. 2020, 132, 86. [Google Scholar] [CrossRef]
- Bakhouch, M.; Al Houari, G.; Daoudi, M.; Kerbal, A.; Yazidi, M. El Michael Addition of Active Methylene Compounds to (Z)-2-Arylidenebenzo[b]Thiophen-3(2H)-Ones. Mediterr. J. Chem. 2015, 4, 9–17. [Google Scholar] [CrossRef]
- Chalkha, M.; Akhazzane, M.; Moussaid, F.Z.; Daoui, O.; Nakkabi, A.; Bakhouch, M.; Chtita, S.; Elkhattabi, S.; Iraqi Housseini, A.; El Yazidi, M. Design, Synthesis, Characterization, in Vitro Screening, Molecular Docking, 3D-QSAR, and ADME-Tox Investigations of Novel Pyrazole Derivatives as Antimicrobial Agents. New J. Chem. 2022, 46, 2747–2760. [Google Scholar] [CrossRef]
- Chalkha, M.; El Moussaoui, A.; Hadda, T.B.; Berredjem, M.; Bouzina, A.; Almalki, F.A.; Saghrouchni, H.; Bakhouch, M.; Saadi, M.; El Ammari, L.; et al. Crystallographic Study, Biological Evaluation and DFT/POM/Docking Analyses of Pyrazole Linked Amide Conjugates: Identification of Antimicrobial and Antitumor Pharmacophore Sites. J. Mol. Struct. 2022, 1252, 131818. [Google Scholar] [CrossRef]
- Chalkha, M.; Nakkabi, A.; Hadda, T.B.; Berredjem, M.; El Moussaoui, A.; Bakhouch, M.; Saadi, M.; El Ammari, L.; Almalki, F.A.; Laaroussi, H.; et al. Crystallographic Study, Biological Assessment and POM/Docking Studies of Pyrazoles-Sulfonamide Hybrids (PSH): Identification of a Combined Antibacterial/Antiviral Pharmacophore Sites Leading to in-Silico Screening the Anti-COVID-19 Activity. J. Mol. Struct. 2022, 1267, 133605. [Google Scholar] [CrossRef]
- Ouyang, G.; Zhang, P.; Xu, G.; Song, B.; Yang, S.; Jin, L.; Xue, W.; Hu, D.; Lu, P.; Chen, Z. Synthesis and Antifungal Bioactivities of 3-Alkylquinazolin- 4-One Derivatives. Molecules 2006, 11, 383–392. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Amaral, J.D.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Synthesis and Evaluation of Spiroisoxazoline Oxindoles as Anticancer Agents. Bioorganic Med. Chem. 2014, 22, 577–584. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. I. The Effect of the Exchange-only Gradient Correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Stecko, S.; Paśniczek, K.; Michel, C.; Milet, A.; Pérez, S.; Chmielewski, M. A DFT Study of 1,3-Dipolar Cycloaddition Reactions of 5-Membered Cyclic Nitrones with α,β-Unsaturated Lactones and with Cyclic Vinyl Ethers: Part 1. Tetrahedron Asymmetry 2008, 19, 1660–1669. [Google Scholar] [CrossRef]
- Hammoudan, I.; Matchi, S.; Bakhouch, M.; Belaidi, S.; Chtita, S. QSAR Modelling of Peptidomimetic Derivatives towards HKU4-CoV 3CLpro Inhibitors against MERS-CoV. Chemistry 2021, 3, 391–401. [Google Scholar] [CrossRef]
- Iron, M.A.; Martin, J.M.L.; Van der Boom, M.E. Cycloaddition Reactions of Metalloaromatic Complexes of Iridium and Rhodium: A Mechanistic DFT Investigation. J. Am. Chem. Soc. 2003, 125, 11702–11709. [Google Scholar] [CrossRef] [PubMed]
- Heydari, S.; Haghdadi, M.; Hamzehloueian, M.; Bosra, H.G. An Investigation of the Regio-, Chemo-, and Stereoselectivity of Cycloaddition Reactions of 2-Phenylsulfonyl-1,3-Butadiene and Its 3-Phenylsulfanyl Derivative: A DFT Study. Struct. Chem. 2021, 32, 1819–1831. [Google Scholar] [CrossRef]
- Zhang, I.Y.; Wu, J.; Xu, X. Extending the Reliability and Applicability of B3LYP. Chem. Commun. 2010, 46, 3057–3070. [Google Scholar] [CrossRef]
- Haghdadi, M.; Nab, N.; Bosra, H.G. DFT Study on the Regio- and Stereo-Selectivity of the Diels-Alder Reaction between a Cycloprop-2-Ene Carboxylate and Some Cyclic 1,3-Dienes. Prog. React. Kinet. Mech. 2016, 41, 193–204. [Google Scholar] [CrossRef]
- Hammoudan, I.; Chtita, S.; Riffi-Temsamani, D. QTAIM and IRC Studies for the Evaluation of Activation Energy on the C=P, C=N and C=O Diels-Alder Reaction. Heliyon 2020, 6, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, E.; Pérez, P.; Domingo, L.R. On the Nature of Parr Functions to Predict the Most Reactive Sites along Organic Polar Reactions. Chem. Phys. Lett. 2013, 582, 141–143. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Roby, O.; Hammoudan, I.; Saddik, R.; Garcia, Y.; Almarhoon, Z.M.; Mabkhot, Y.N. Kinetics, Thermodynamics, Equilibrium, Surface Modelling, and Atomic Absorption Analysis of Selective Cu(Ii) Removal from Aqueous Solutions and Rivers Water Using Silica-2-(Pyridin-2-Ylmethoxy)Ethan-1-Ol Hybrid Material. RSC Adv. 2022, 12, 611–625. [Google Scholar] [CrossRef]
- Laidler, K.J. The Development of the Arrhenius Equation. J. Chem. Educ. 1984, 61, 494–498. [Google Scholar] [CrossRef]
- Boshaala, A.; Said, M.A.; Assirey, E.A.; Alborki, Z.S.; AlObaid, A.A.; Zarrouk, A.; Warad, I. Crystal Structure, MEP/DFT/XRD, Thione ⇔ Thiol Tautomerization, Thermal, Docking, and Optical/TD-DFT Studies of (E)-Methyl 2-(1-Phenylethylidene)-Hydrazinecarbodithioate Ligand. J. Mol. Struct. 2021, 1238, 130461. [Google Scholar] [CrossRef]
- Dupuis, M.; Chen, S.; Raugei, S.; Dubois, D.L.; Bullock, R.M. Comment on “New Insights in the Electrocatalytic Proton Reduction and Hydrogen Oxidation by Bioinspired Catalysts: A DFT Investigation”. J. Phys. Chem. A 2011, 115, 4861–4865. [Google Scholar] [CrossRef] [PubMed]
- Wick, C.R.; Clark, T. On Bond-Critical Points in QTAIM and Weak Interactions. J. Mol. Model. 2018, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Liu, K.-C.; Shelton, B.R.; Howe, R.K. A Particularly Convenient Preparation of Benzohydroximinoyl Chlorides (Nitrile Oxide Precursors). J. Org. Chem. 1980, 45, 3916–3918. [Google Scholar] [CrossRef]
- Talha, A.; Favreau, C.; Bourgoin, M.; Robert, G.; Auberger, P.; EL Ammari, L.; Saadi, M.; Benhida, R.; Martin, A.R.; Bougrin, K. Ultrasound-Assisted One-Pot Three-Component Synthesis of New Isoxazolines Bearing Sulfonamides and Their Evaluation against Hematological Malignancies. Ultrason. Sonochem. 2021, 78, 105748. [Google Scholar] [CrossRef]
- Lobo, M.M.; Viau, C.M.; Dos Santos, J.M.; Bonacorso, H.G.; Martins, M.A.P.; Amaral, S.S.; Saffi, J.; Zanatta, N. Synthesis and Cytotoxic Activity Evaluation of Some Novel 1-(3-(Aryl-4,5-Dihydroisoxazol-5-Yl)Methyl)-4-Trihalomethyl-1H-Pyrimidin-2-Ones in Human Cancer Cells. Eur. J. Med. Chem. 2015, 101, 836–842. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. Global and Local Reactivity Indices for Electrophilic/Nucleophilic Free Radicals. Org. Biomol. Chem. 2013, 11, 4350–4358. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the Local Reactivity in Polar Organic Reactions through Electrophilic and Nucleophilic Parr Functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Schmider, H.L.; Becke, A.D. Chemical Content of the Kinetic Energy Density. J. Mol. Struct. THEOCHEM 2000, 527, 51–61. [Google Scholar] [CrossRef]










| Entry | R | Formula (M. g/mol) | M.p. (°C) | Yield a (%) | NMR-1H (ppm) | 13C-NMR (ppm) | IR (cm−1) | HRMS(m/z) [M+H]+ |
|---|---|---|---|---|---|---|---|---|
| N–CH2 CH2(isoxazoline) CH(isoxazoline) | N-CH2 CH2 CH | C=O C=N | ||||||
| 4a | 4-Br | C18H14N3O2Br (383.03) | 194 | 96 | 4.05 (dd, 1H); 4.44 (dd, 1H) 3.20 (dd, 1H); 3.52 (dd, 1H) 5.11–−5.20 (m, 1H) | 49.16 37.88 78.63 | 1670 1610 | 384.03367 |
| 4b | 4-Cl | C18H14N3O2Cl (339.08) | 176 | 82 | 4.08 (dd, 1H); 4.46 (dd, 1H) 3.23 (dd, 1H); 3.54 (dd, 1H) 5.12–5.22 (m, 1H) | 49.15 37.95 78.63 | 1672 1598 | 340.08417 |
| 4c | 4-OCH3 | C19H17N3O3 (335.13) | 210 | 74 | 4.05 (dd, 1H); 4.46 (dd, 1H) 3.19 (dd, 1H); 3.53 (dd, 1H) 5.10–5.19 (m, 1H) | 49.19 37.96 78.59 | 1666 1600 | 336.13332 |
| 4d | 4-NO2 | C18H14N4O4 (350.1) | 236 | 84 | 4.17 (dd, 1H); 4.47 (dd, 1H) 3.31 (dd, 1H); 3.59 (dd, 1H) 5. 21–5.32 (m, 1H) | 49.16 37.88 78.63 | 1662 1610 | 351.10835 |
| 4e | 4-CH3 | C19H17N3O2 (319.13) | 174 | 92 | 4.01 (dd, 1H); 4.47 (dd, 1H) 3.21 (dd, 1H); 3.56 (dd, 1H) 5. 09–5.18 (m, 1H) | 49.30 38.25 78.07 | 1674 1612 | 320.13944 |
| 4f | H | C18H15N3O2 (305.12) | 166 | 59 | 4.45 (dd, 1H); 4.04 (dd, 1H) 3.34 (dd, 1H); 3.58 (dd, 1H) 5. 11–5.20 (m, 1H) | 49.26 38.13 78.28 | 1662 1608 | 306.12318 |
| 4g | 2-Cl | C18H14N3O2Cl (339.08) | 168 | 91 | 4.12 (dd, 1H); 4.43 (dd, 1H) 3.44 (dd, 1H); 3.68 (dd, 1H) 5. 15–5.24 (m, 1H) | 49.09 40.51 78.83 | 1670 1608 | 340.08428 |
| BCP | Bonding | ρ | ∇²ρ | |
|---|---|---|---|---|
| TS | 67 | C16–C17 | 0.53 | 0.55 |
| 82 | C13–O14 | 0.33 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhazi, Y.; Chalkha, M.; Nakkabi, A.; Hammoudan, I.; Akhazzane, M.; Bakhouch, M.; Chtita, S.; El Yazidi, M. Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study. Chemistry 2022, 4, 969-982. https://doi.org/10.3390/chemistry4030066
Rhazi Y, Chalkha M, Nakkabi A, Hammoudan I, Akhazzane M, Bakhouch M, Chtita S, El Yazidi M. Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study. Chemistry. 2022; 4(3):969-982. https://doi.org/10.3390/chemistry4030066
Chicago/Turabian StyleRhazi, Yassine, Mohammed Chalkha, Asmae Nakkabi, Imad Hammoudan, Mohamed Akhazzane, Mohamed Bakhouch, Samir Chtita, and Mohamed El Yazidi. 2022. "Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study" Chemistry 4, no. 3: 969-982. https://doi.org/10.3390/chemistry4030066
APA StyleRhazi, Y., Chalkha, M., Nakkabi, A., Hammoudan, I., Akhazzane, M., Bakhouch, M., Chtita, S., & El Yazidi, M. (2022). Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study. Chemistry, 4(3), 969-982. https://doi.org/10.3390/chemistry4030066