Trinuclear and Cyclometallated Organometallic Dinuclear Pt-Pyrazolato Complexes: A Combined Experimental and Theoretical Study
Abstract
:1. Introduction
2. Results and Discussion
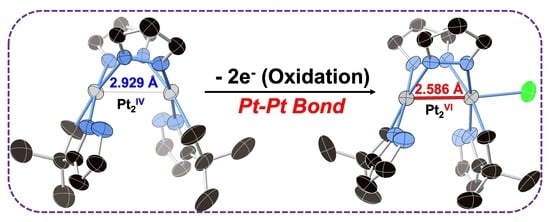
2.1. Synthesis and Characterization
2.2. Computational Studies
3. Materials, Methods, and Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fackler, J.P., Jr. Metal-metal bond formation in the oxidative addition to dinuclear gold(I) species. Implications from dinuclear and trinuclear gold chemistry for the oxidative addition process generally. Polyhedron 1997, 16, 1–17. [Google Scholar] [CrossRef]
- Fackler, J.P., Jr. Forty-Five Years of Chemical Discovery Including a Golden Quarter-Century. Inorg. Chem. 2002, 41, 6959–6972. [Google Scholar] [CrossRef] [PubMed]
- Schenck, T.G.; Milne, C.R.C.; Sawyer, J.F.; Bosnich, B. Bimetallic reactivity. Oxidative-addition and reductive-elimination reactions of rhodium and iridium bimetallic complexes. Inorg. Chem. 1999, 24, 2338–2344. [Google Scholar] [CrossRef]
- Laguna, A.; Laguna, M. Coordination chemistry of gold(II) complexes. Coord. Chem. Rev. 1999, 193–195, 837–856. [Google Scholar] [CrossRef]
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. (Eds.) Multiple Bonds between Metal Atoms, 3rd ed.; Springer Science and Business Media, Inc.: New York, NY, USA, 2005; Chapter 14; pp. 636–661. [Google Scholar]
- Matsumoto, K.; Ochiai, M. Organometallic chemistry of platinum-blue derived platinum III dinuclear complexes. Coord. Chem. Rev. 2002, 231, 229–238. [Google Scholar] [CrossRef]
- Murray, H.H., III; Briggs, D.A.; Garzón, G.; Raptis, R.G.; Porter, L.C.; Fackler, J.P., Jr. Structural Characterization of a Linear [Au···Pt···Au] Complex, Au2Pt(CH2P(S)Ph2)4, and its Oxidized Linear Metal-Metal Bonded [Au-Pt-Au] Product, Au2Pt(CH2P(S)Ph2)4Cl2. Organometallics 1987, 6, 1992–1995. [Google Scholar] [CrossRef]
- Yang, G.; Matínez, J.R.; Raptis, R.G. Dinuclear gold(III) pyrazolato complexes—Synthesis, structural characterization and transformation to their trinuclear gold(I) and gold(I/III) analogues. Inorg. Chim. Acta 2009, 362, 1546–1552. [Google Scholar] [CrossRef]
- Yang, G.; Raptis, R.G. Oxidation of gold(I) pyrazolates by aqua regia. X-Ray crystal structures of the first examples of trinuclear AuIII3 and AuIAuIII2 pyrazolato complexes. J. Chem. Soc. Dalton Trans. 2002, 21, 3936–3938. [Google Scholar] [CrossRef]
- Raptis, R.G.; Fackler, J.P., Jr. The synthesis and crystal structure of a mixed-valence, digold(I)/gold(III), pyrazolato complex stable in aqua regia. The x-ray photoelectron study of homo- and heterovalent gold-pyrazolato trimers. Inorg. Chem. 1990, 29, 5003–5006. [Google Scholar] [CrossRef]
- Raptis, R.G.; Murray, H.H.; Fackler, J.P., Jr. The structure of [Au-µ-{3,5-(C6H5)2C3HN2}]3Cl2: A trinuclear mixed-valence gold pyrazolate complex. Acta Crystallogr. 1988, C44, 970–973. [Google Scholar] [CrossRef]
- Teets, T.S.; Nocera, D.G. Halogen Photoreductive Elimination from Gold(III) Centers. J. Am. Chem. Soc. 2009, 131, 7411–7420. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.J.; Rendina, L.M.; Puddephatt, R.J. A strategy for synthesis of large gold rings. Chem. Commun. 1996, 11, 1281–1282. [Google Scholar] [CrossRef]
- Arnal, L.; Escudero, D.; Fuertes, S.; Martin, A.; Sicilia, V. High-Valent Pyrazolate-Bridged Platinum Complexes: A Joint Experimental and Theoretical Study. Inorg. Chem. 2022, 61, 12559–12569. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-H.; Chi, Y.; Chen, Y.-L.; Liu, C.-S.; Ching, W.-L.; Carty, A.J.; Peng, S.-M.; Lee, G.-H. A Study of Unsaturated Pyrazolate-Bridged Diruthenium Carbonyl Complexes. Organometallics 2002, 21, 4735–4742. [Google Scholar] [CrossRef]
- Tejel, C.; Ciriano, M.A.; Lopez, J.A.; Lahoz, F.J.; Oro, L.A. New Perspective on the Formation and Reactivity of Metal−Metal-Bonded Dinuclear Rhodium and Iridium Complexes. Organometallics 1997, 16, 4718–4727. [Google Scholar] [CrossRef]
- Coleman, A.W.; Eadie, D.T.; Stobart, S.R.; Zaworotko, M.J.; Atwood, J.L. Pyrazolyl-bridged iridium dimers. 2. Contrasting modes of two-center oxidative addition to a bimetallic system and reductive access to the starting complex: Three key diiridium structures representing short nonbonding and long and short bonding metal-metal interactions. J. Am. Chem. Soc. 1982, 104, 922–923. [Google Scholar]
- Umakoshi, K.; Kojima, T.; Kim, Y.H.; Onishi, M.; Nakao, Y.; Sasaki, S. Deep Blue Mixed-Valent PtIIIPtIIIPtII Complex [Pt3Br2(μ-pz)6] (pz=Pyrazolate) Showing Valence-Detrapping Behavior in Solution. Chem. Eur. J. 2006, 12, 6521–6727. [Google Scholar] [CrossRef]
- Horiuchi, S.; Umakoshi, K. Recent advances in pyrazolato-bridged homo- and heterometallic polynuclear platinum and palladium complexes. Coord. Chem. Rev. 2023, 476, 214924. [Google Scholar] [CrossRef]
- Baran, P.; Marrero, C.M.; Pérez, S.; Raptis, R.G. Stepwise, ring-closure synthesis and characterization of a homoleptic palladium(ii)-pyrazolato cyclic trimer. Chem. Commun. 2002, 9, 1012–1013. [Google Scholar] [CrossRef]
- Mohamed, A.A. Advances in the coordination chemistry of nitrogen ligand complexes of coinage metals. Coord. Chem. Rev. 2010, 254, 1918–1947. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Lu, H.-L. From Metal-Metal Bonding to Supra-Metal-Metal Bonding Directed Self-Assembly: Supramolecular Architectures of Group 10 and 11 Metals with Ligands from Mono- to Poly-Pyrazoles. Isr. J. Chem. 2019, 59, 166–183. [Google Scholar] [CrossRef]
- Galassi, R.; Rawashdeh-Omary, M.A.; Dias, H.V.R.; Omary, M.A. Homoleptic Cyclic Trinuclear d10 Complexes: From Self-Association via Metallophilic and Excimeric Bonding to the Breakage Thereof via Oxidative Addition, Dative Bonding, Quadrupolar, and Heterometal Bonding Interactions. Comm. Inorg. Chem. 2019, 39, 287–348. [Google Scholar] [CrossRef] [Green Version]
- Canty, A.J.; Honeyman, C.T. Cyclometallation of polydentate ligands containing pyrazole groups, including the synthesis of platinum(IV) complexes with tripodal [N^C^N]- ligand systems. J. Organomet. Chem. 1990, 387, 247–263. [Google Scholar] [CrossRef]
- Albrecht, M. Cyclometalation Using d-Block Transition Metals: Fundamental Aspects and Recent Trends. Chem. Rev. 2010, 110, 576–623. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, L.; Urriolabeitia, E.P. Cyclometallation of Heterocycles: A Reliable Strategy for Selective Functionalization. Comm. Inorg. Chem. 2012, 33, 55–85. [Google Scholar] [CrossRef]
- Lu, W.; Mi, B.-X.; Chan, M.C.W.; Hui, Z.; Che, C.-M.; Zhu, N.; Lee, S.-T. Light-Emitting Tridentate Cyclometalated Platinum(II) Complexes Containing ó-Alkynyl Auxiliaries: Tuning of Photo- and Electrophosphorescence. J. Am. Chem. Soc. 2004, 126, 4958–4971. [Google Scholar] [CrossRef]
- Koo, C.K.; Ho, Y.M.; Chow, C.-F.; Lam, M.H.-W.; Lau, T.-C.; Wong, W.-Y. Synthesis and Spectroscopic Studies of Cyclometalated Pt(II) Complexes Containing a Functionalized Cyclometalating Ligand, 2-Phenyl-6-(1H-pyrazol-3-yl)-pyridine. Inorg. Chem. 2007, 46, 3606–3612. [Google Scholar] [CrossRef]
- Bossi, A.; Rausch, A.F.; Leitl, M.J.; Czerwieniec, R.; Whited, M.T.; Djurovich, P.I.; Yersin, H.; Thompson, M.E. Photophysical Properties of Cyclometalated Pt(II) Complexes: Counterintuitive Blue Shift in Emission with an Expanded Ligand π System. Inorg. Chem. 2013, 52, 12403–12415. [Google Scholar] [CrossRef]
- Hashiguchi, B.G.; Bischof, S.M.; Konnick, M.M.; Periana, R.A. Designing Catalysts for Functionalization of Unactivated C-H Bonds Based on the CH Activation Reaction. Acc. Chem. Res. 2012, 45, 885–898. [Google Scholar] [CrossRef]
- Lersch, M.; Tilset, M. Mechanistic Aspects of C-H Activation by Pt Complexes. Chem. Rev. 2005, 105, 2471–2526. [Google Scholar] [CrossRef]
- Burger, W.; Strähle, J.Z. Pyrazolate and tetrazolate as bridging ligands in [Pt(pz)2]3, [Pt(pz)2]∞, and [Pt(tz)2]∞. Crystal structure of [Pt(pz)2]3. Anorg. Allg. Chem. 1985, 529, 111–117. [Google Scholar] [CrossRef]
- Umakoshi, K.; Yamauchi, Y.; Nakamiya, K.; Yamasaki, M.; Kawano, H.; Onishi, M. Pyrazolato-Bridged Polynuclear Palladium and Platinum Complexes: Synthesis, Structure, and Reactivity. Inorg. Chem. 2003, 42, 3907–3916. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ma, B.; Li, J.; Djurovich, P.I.; Yousufuddin, M.; Bau, R.; Thompson, M.E. Synthetic Control of Pt···Pt Separation and Photophysics of Binuclear Platinum Complexes. J. Am. Chem. Soc. 2005, 127, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Baxter, L.M.A.; Heath, G.A.; Raptis, R.G.; Willis, A.C. Synthesis and Characterization of a Diplatinum(III)-Tetrakis(α-dioximato) Complex Containing an Unsupported Metal-Metal Bond. J. Am. Chem. Soc. 1992, 114, 6944–6946. [Google Scholar] [CrossRef]
- Cini, R.; Fanizzi, F.P.; Natile, G. Synthesis and x-ray structural characterization of the first unbridged diplatinum(III) compound: Bis[bis(1-imino-1-hydroxy-2,2-dimethylpropane)trichloroplatinum(III)]. J. Am. Chem. Soc. 1991, 113, 7805–7806. [Google Scholar] [CrossRef]
- Tadashi, Y.; Osamu, K.; Tasuku, I. An Unbridged Platinum(III) Dimer with Added Chloro Ligands in Equatorial Sites, [Pt2Cl2(phpy)4] (Hphpy = phenylpyridine), Synthesized by an Oxidation with Aurous Complex. Chem. Lett. 2004, 33, 190–191. [Google Scholar]
- Lippert, B. Impact of Cisplatin on the recent development of Pt coordination chemistry: A case study. Coord. Chem. Rev. 1999, 182, 263–295. [Google Scholar] [CrossRef]
- Vedernikov, A.N. Trivalent and Tetravalent Palladium and Platinum Organometallic Complexes. In Comprehensive Organimetallic Chemistry IV; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–53. [Google Scholar]
- Ramanchenko, A.; Likhatski, M.; Mikhlin, Y. X-ray Photoelectron Spectroscopy (XPS) Study of the Products Formed on Sulfide Minerals Upon the Interaction with Aqueous Platinum (IV Chloride Complexes). Minerals 2018, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Papadia, P.; Micoli, K.; Barbanente, A.; Ditaranto, N.; Hoeschele, J.D.; Natile, G.; Marzano, C.; Gandin, V.; Margiotta, N. Platinum(IV) Complexes of trans-1,2 diamino-4-cyclohexene: Prodrugs A_ording an Oxaliplatin Analogue that Overcomes Cancer Resistance. Inter. J. Mol. Sci. 2020, 21, 2325. [Google Scholar] [CrossRef] [Green Version]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Popelier, P.L.A. Atoms in Molecules: An Introduction; Prentice Hall: Upple Saddle River, NJ, USA, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J. (Eds.) The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 2nd ed.; Pergamon Press: New York, NY, USA, 1987. [Google Scholar]
- Data Collection: SMART-NT Software Reference Manual, version 5.0. ed. Bruker AXS, Inc.: Madison, WI, USA, 1998.
- Data Reduction: SAINT-NT Software Reference Manual, version 4.0. ed. Bruker AXS, Inc.: Madison, WI, USA, 1996.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- de Berrêdo, R.C.; Jorge, F.E. All-electron double zeta basis sets for platinum: Estimating scalar relativistic effects on platinum (II) anticancer drugs. J. Mol. Struc. (Theochem) 2010, 961, 107–112. [Google Scholar] [CrossRef]
- Biegler-König, F. AIM2000, Version 1.0; University of Applied Sciences: Bielefeld, Germany, 2000. [Google Scholar]
- Horiuchi, S.; Moon, S.; Ito, A.; Tessarolo, J.; Sakuda, E.; Arikawa, Y.; Clever, G.H.; Umakoshi, K. Multinuclear Ag Clusters Sandwiched by Pt Complex Units: Fluxional Behavior and Chiral-at-Cluster Photoluminescence. Angew. Chem. Int. Ed. 2021, 60, 10654–10660. [Google Scholar] [CrossRef]
- Horiuchi, S.; Yang, Y.; Ueda, M.; Sakuda, E.; Arikawa, Y.; Umakoshi, K. Rational Synthesis of an Unsymmetric Pt Complex Unit Having Two Kinds of Pyrazolate Ligands, Elucidating Steric and Electronic Effects of Pyrazolate Ligands in Pt-Ag Sandwich Complexes. Eur. J. Inorg. Chem. 2022, 2022, e202200497. [Google Scholar] [CrossRef]
- Roy, S.; Lopez, A.A.; Yarnell, J.E.; Castellano, F.N. Metal−Metal-to-Ligand Charge Transfer in Pt(II) Dimers Bridged by Pyridyl and Quinoline Thiols. Inorg. Chem. 2022, 61, 121–130. [Google Scholar] [CrossRef]
- Park, H.J.; Boelke, C.L.; Cheong, P.H.-Y.; Hwang, D.-H. Dinuclear Pt(II) Complexes with Red and NIR Emission Governed by Ligand Control of the Intramolecular Pt−Pt Distance. Inorg. Chem. 2022, 61, 5178–5183. [Google Scholar] [CrossRef]
- Xue, M.; Lam, T.-L.; Cheng, G.; Liu, W.; Low, K.-H.; Du, L.; Xu, S.; Hung, F.-F.; Phillips, D.L.; Che, C.-M. Exceedingly Stable Luminescent Dinuclear Pt(II) Complexes with Ditopic Formamidinate Bridging Ligands for High-Performance Red and Deep-Red OLEDs with LT97 up to 2446 h at 1000 cd m−2. Adv. Optical Mater. 2022, 10, 2200741. [Google Scholar] [CrossRef]
- Lai, S.-W.; Chan, M.C.W.; Cheung, K.-K.; Peng, S.-M.; Che, C.-M. Synthesis of Organoplatinum Oligomers by Employing N-Donor Bridges with Predesigned Geometry: Structural and Photophysical Properties of Luminescent Cyclometalated Platinum(II) Macrocycles. Organometallics 1999, 18, 3991–3997. [Google Scholar] [CrossRef]
- Brown-Xu, S.E.; Kelley, M.S.; Fransted, K.A.; Chakraborty, A.; Schatz, G.C.; Castellano, F.N.; Chen, L.X. Tunable Excited-State Properties and Dynamics as a Function of Pt-Pt Distance in Pyrazolate-Bridged Pt(II) Dimers. J. Phys. Chem. A 2016, 120, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Deaton, J.C.; Haefele, A.; Castellano, F.N. Charge-Transfer and Ligand-Localized Photophysics in Luminescent Cyclometalated Pyrazolate-Bridged Dinuclear Platinum(II) Complexes. Organometallics 2013, 32, 3819–3829. [Google Scholar] [CrossRef]









| 1 | 2 | 3 | |
|---|---|---|---|
| Pt-Pt | 3.0355(5)–3.0758(6) 3.110, 3.114, 3.117 | 2.9290(5) 3.031 | 2.584(3), 2.586(2) 2.640 |
| Pt-N(μ-pz) | 2.006(5)–2.023(5) 2.044–2.047 | 2.026(6)–2.157(6) 2.060–2.235 | 2.026(7)–2.222(7) 2.068–2.312 |
| Pt-N(κ2-pzH) | - | 1.961(8) 1.995, 1.996 | 1.973(7)–2.015(7) 2.000, 2.017 |
| Pt-C | - | 2.048(10), 2.034(10) 2.074, 2.077 | 2.060(8)–2.078(9) 2.089, 2.100 |
| Pt-Cl | - | - | 2.352(3), 2.346(3) 2.434 |
| Pt-N-N | 113.4(4)–116.3(4) 113.3–115.7 | 108.9(5)–130.1(6) 110.9–112.6 | 101.7(5)–133.7(6) 101.8–111.6 |
| N-Pt-N (cis-pz) | 86.6(2)–93.0(2) 81.8–89.2 | 89.2(3)–89.3(3) 88.2 | 87.6(3)–89.4(3) 88.1–91.1 |
| N-Pt-N (trans) | 170.3(2)–172.8(2) 168.2–175.7 | 172.2(3)–172.3(3) 175.3, 175.7 | 168.1(3)–174.5(3) 170.2, 175.0 |
| N-Pt-C | - | 79.3(4), 80.1(4) 79.2, 79.3 | 80.0(3)–81.2(3) 80.5, 80.6 |
| Pt-Pt-Cl | - | - | 174.72(8), 174.91(7) 173.556 |
| pz-Pt-Pt-pz | 96.9–121.7 (average 110.6) 94.2, 98.1, 103.4 | 102.6 105.3 | 92.1, 95.9 92.4 |
| Compound | 4f7/2 (eV) | 4f5/2 (eV) |
|---|---|---|
| 2 | 72.6 | 75.4 |
| 3 (PtN3CCl) | 73.1 | 76.4 |
| 3 (PtN3C) | 74.4 | 77.7 |
| H2PtCl6 | 75.2 a | 78.6 a |
| [Pt(oxa)(OH)2(dachex)] | 75.6 a | 79.0 a |
| 1·0.5CH3COCH3 | 2 | 3·2H2O | |
|---|---|---|---|
| Formula | C25.5H33N12O0.5Pt3 | C44H76N8Pt2 | C44H41ClN8O2Pt2 |
| Crystal size, mm3 | 0.08 × 0.06 × 0.05 | 0.40 × 0.30 × 0.20 | 0.22 × 0.18 × 0.10 |
| fw | 1100.91 | 1107.30 | 1173.75 |
| Space group | P-1 (No. 2) | P212121 (No. 19) | P-1 (No. 2) |
| a, Å | 13.734(3) | 12.373(1) | 11.643(9) |
| b, Å | 13.837(2) | 18.696(2) | 21.040(2) |
| c, Å | 17.734(3) | 21.343(2) | 23.064(13) |
| α, ° | 74.55(1) | 90 | 64.77(7) |
| β, ° | 81.87(1) | 90 | 83.98(6) |
| γ, ° | 74.81(1) | 90 | 82.48(8) |
| V, Å3 | 3125.5(9) | 4937.2(8) | 5059(7) |
| Z | 2 | 4 | 4 |
| T, K | 298(2) | 298(2) | 298(2) |
| ρcalcd, g cm−3 | 2.34 | 1.49 | 1.54 |
| reflctns collected/2θmax | 11,453/51.00 | 29,004/52.00 | 28,262/50.00 |
| Unique reflctns/I > 2σ(I) | 12,088/10,030 | 9589/8743 | 17,509/13,447 |
| No. of params/restraints | 753/0 | 513/46 | 1071/0 |
| μ(Mo Kα), mm−1 | 13.433 | 5.696 | 5.617 |
| F(000) | 2032 | 2208 | 2336 |
| R1 a/All data | 0.0285/0.0389 | 0.0311/0.0402 | 0.0471/0.0773 |
| wR2 b (I > 2σ(I)) | 0.0663 | 0.0698 | 0.1245 |
| Goodness of fit c | 1.057 | 1.184 | 1.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Li, F.; Zhao, H.; Chakraborty, I.; Chen, Z.; Raptis, R.G. Trinuclear and Cyclometallated Organometallic Dinuclear Pt-Pyrazolato Complexes: A Combined Experimental and Theoretical Study. Chemistry 2023, 5, 187-200. https://doi.org/10.3390/chemistry5010016
Shi Z, Li F, Zhao H, Chakraborty I, Chen Z, Raptis RG. Trinuclear and Cyclometallated Organometallic Dinuclear Pt-Pyrazolato Complexes: A Combined Experimental and Theoretical Study. Chemistry. 2023; 5(1):187-200. https://doi.org/10.3390/chemistry5010016
Chicago/Turabian StyleShi, Zhichun, Fengyu Li, Hong Zhao, Indranil Chakraborty, Zhongfang Chen, and Raphael G. Raptis. 2023. "Trinuclear and Cyclometallated Organometallic Dinuclear Pt-Pyrazolato Complexes: A Combined Experimental and Theoretical Study" Chemistry 5, no. 1: 187-200. https://doi.org/10.3390/chemistry5010016
APA StyleShi, Z., Li, F., Zhao, H., Chakraborty, I., Chen, Z., & Raptis, R. G. (2023). Trinuclear and Cyclometallated Organometallic Dinuclear Pt-Pyrazolato Complexes: A Combined Experimental and Theoretical Study. Chemistry, 5(1), 187-200. https://doi.org/10.3390/chemistry5010016