Conversion of Ethanol to Butadiene over Binary MgO-SiO2 Mixed Oxides Prepared by the Ammonia Evaporation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
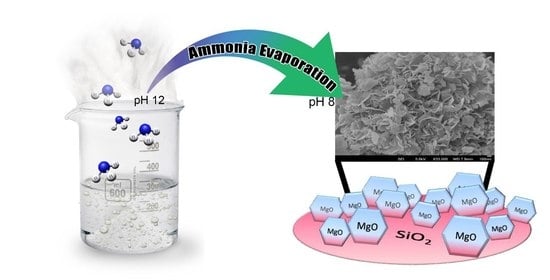
2.2. Catalyst Preparation
2.3. Catalyst Characterisation
2.4. Catalytic Activity
3. Results and Discussion
3.1. Textural Properties of MgO-SiO2
3.2. Acid-Base Properties
3.3. Catalytic Activity for Ethanol to Butadiene (ETB)
3.3.1. Variation of Mg/Si Ratio, Reaction Temperature and Flow Rates
3.3.2. Effect of Synthesis Methods
3.3.3. Stability of Ammonia-Evaporated MgO-SiO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdussalam-Mohammed, W.; Ali, A.; Errayes, A. Green chemistry: Principles, applications, and disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar]
- Anastas, P.T.; Zimmerman, J.B. The periodic table of the elements of green and sustainable chemistry. Green Chem. 2019, 21, 6545–6566. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.N.; Wristers, J.P. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2004; pp. 365–392. [Google Scholar]
- Global Butadiene Market Overview (2014–2025). Available online: https://prismaneconsulting.com/blog_details/101/Global-Butadiene-Market-Overview-(2014-2025) (accessed on 10 January 2021).
- Morrow, N.L. The industrial production and use of 1, 3-butadiene. Environ. Health Perspect. 1990, 86, 7–8. [Google Scholar] [CrossRef]
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wristers, J. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2000; pp. 365–392. [Google Scholar]
- Asia/World Energy Outlook 2013. Available online: https://eneken.ieej.or.jp/en/whatsnew/413.html (accessed on 23 January 2022).
- Kim, S.; Jeong, S.; Heo, E. Effects of the shale boom on ethylene and propylene prices. Energy Sources Part B Econ. Plan. Policy 2019, 14, 49–66. [Google Scholar] [CrossRef]
- Matar, S.; Hatch, L.F. Chemistry of Petrochemical Processes; Butterworth-Heinemann: Woburn, UK, 2001. [Google Scholar] [CrossRef]
- Angili, T.S.; Grzesik, K.; Rödl, A.; Kaltschmitt, M. Life Cycle Assessment of Bioethanol Production: A Review of Feedstock, Technology and Methodology. Energies 2021, 14, 2939. [Google Scholar] [CrossRef]
- Karimi, S.; Karri, R.R.; Yaraki, M.T.; Koduru, J.R. Processes and separation technologies for the production of fuel-grade bioethanol: A review. Environ. Chem. Lett. 2021, 19, 2873–2890. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Wang, X.; Lü, X. A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Pomalaza, G.; Ponton, P.A.; Capron, M.; Dumeignil, F.Y. Ethanol-to-butadiene: The reaction and its catalysts. Catal. Sci. Technol. 2020, 10, 4860–4911. [Google Scholar] [CrossRef]
- Bin Samsudin, I.; Zhang, H.; Jaenicke, S.; Chuah, G. Recent Advances in Catalysts for the Conversion of Ethanol to Butadiene. Chem. Asian J. 2020, 15, 4199–4214. [Google Scholar] [CrossRef] [PubMed]
- Kyriienko, P.I.; Larina, O.V.; Soloviev, S.O.; Orlyk, S.M. Catalytic Conversion of Ethanol Into 1,3-Butadiene: Achievements and Prospects: A Review. Theor. Exp. Chem. 2020, 56, 213–242. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Bandinelli, C.; Vozniuk, O.; Chieregato, A.; Malmusi, A.; Recchi, C.; Cavani, F. An analysis of the chemical, physical and reactivity features of MgO–SiO2 catalysts for butadiene synthesis with the Lebedev process. Green Chem. 2016, 18, 1653–1663. [Google Scholar] [CrossRef]
- Huang, X.; Men, Y.; Wang, J.; An, W.; Wang, Y. Highly active and selective binary MgO–SiO2 catalysts for the production of 1, 3-butadiene from ethanol. Catal. Sci. Technol. 2017, 7, 168–180. [Google Scholar] [CrossRef]
- Lewandowski, M.; Babu, G.S.; Vezzoli, M.; Jones, M.D.; Owen, R.E.; Mattia, D.; Plucinski, P.; Mikolajska, E.; Ochenduszko, A.; Apperley, D.C. Investigations into the conversion of ethanol to 1,3-butadiene using MgO:SiO2 supported catalysts. Catal. Commun. 2014, 49, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.-H.; Ramirez, A.; Shoinkhorova, T.; Mukhambetov, I.; Abou-Hamad, E.; Telalovic, S.; Gascon, J.; Ruiz-Martínez, J. The Importance of Thermal Treatment on Wet-Kneaded Silica–Magnesia Catalyst and Lebedev Ethanol-to-Butadiene Process. Nanomaterials 2021, 11, 579. [Google Scholar] [CrossRef]
- Szabó, B.; Novodárszki, G.; Pászti, Z.; Domján, A.; Valyon, J.; Hancsók, J.; Barthos, R. MgO–SiO2 Catalysts for the Ethanol to Butadiene Reaction: The Effect of Lewis Acid Promoters. ChemCatChem 2020, 12, 5686–5696. [Google Scholar] [CrossRef]
- Reschetilowski, W.; Hauser, M.; Alscher, F.; Klauck, M.; Kalies, G. Studies on the Binary MgO/SiO2 Mixed Oxide Catalysts for the Conversion of Ethanol to 1,3-Butadiene. Catalysts 2020, 10, 854. [Google Scholar] [CrossRef]
- Abdulrazzaq, H.T.; Rahmani Chokanlu, A.; Frederick, B.G.; Schwartz, T.J. Reaction kinetics analysis of ethanol dehydrogenation catalyzed by MgO–SiO2. ACS Catal. 2020, 10, 6318–6331. [Google Scholar] [CrossRef]
- Chung, S.-H.; Angelici, C.; Hinterding, S.O.; Weingarth, M.; Baldus, M.; Houben, K.; Weckhuysen, B.M.; Bruijnincx, P.C. Role of Magnesium Silicates in Wet-Kneaded Silica–Magnesia Catalysts for the Lebedev Ethanol-to-Butadiene Process. ACS Catal. 2016, 6, 4034–4045. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, B.; Tan, T. Conversion of Ethanol and Acetaldehyde to Butadiene over MgO–SiO2 Catalysts: Effect of Reaction Parameters and Interaction between MgO and SiO2 on Catalytic Performance. ACS Sustain. Chem. Eng. 2017, 5, 722–733. [Google Scholar] [CrossRef]
- Angelici, C.; Velthoen, M.E.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials. Catal. Sci. Technol. 2015, 5, 2869–2879. [Google Scholar] [CrossRef] [Green Version]
- Angelici, C.; Velthoen, M.E.Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Effect of Preparation Method and CuO Promotion in the Conversion of Ethanol into 1,3-Butadiene over SiO2-MgO Catalysts. Chemsuschem 2014, 7, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Kvisle, S.; Agüero, A.; Sneeden, R.P.A. Transformation of ethanol into 1,3-butadiene over magnesium oxide/silica catalysts. Appl. Catal. 1988, 43, 117–131. [Google Scholar] [CrossRef]
- Niiyama, H.; Morii, S.; Echigoya, E. Butadiene Formation from Ethanol over Silica-Magnesia Catalysts. Bull. Chem. Soc. Jpn. 1972, 45, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, R.; Akimoto, T.; Tanabe, K. Pronounced catalytic activity and selectivity of MgO–SiO2–Na2O for synthesis of buta-1, 3-diene from ethanol. J. Chem. Soc. Chem. Commun. 1985, 22, 1613–1614. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K. Role of Water in the Mechanochemical Reactions of MgO–SiO2 Systems. J. Solid State Chem. 1998, 138, 169–177. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Hu, J.; Tang, Y.; Niu, Y.; Wei, J.; Yu, Q. Characterization of reaction products and reaction process of MgO–SiO2–H2O system at room temperature. Constr. Build. Mater. 2014, 61, 252–259. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K. Formation of Layered Magnesium Silicate during the Aging of Magnesium Hydroxide-Silica Mixtures. J. Am. Ceram. Soc. 1998, 81, 754–756. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Ammonia-Evaporation-Induced Synthetic Method for Metal (Cu, Zn, Cd, Ni) Hydroxide/Oxide Nanostructures. Chem. Mater. 2008, 20, 567–576. [Google Scholar] [CrossRef]
- Chen, L.-F.; Guo, P.-J.; Qiao, M.-H.; Yan, S.-R.; Li, H.-X.; Shen, W.; Xu, H.-L.; Fan, K.-N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Han, X.; Cao, Y.; He, P.; Li, H. Selective hydrogenation of ethylene carbonate to methanol and ethylene glycol over Cu/SiO2 catalysts prepared by ammonia evaporation method. Int. J. Hydrog. Energy 2017, 42, 2144–2156. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Fan, W.; Wang, J.; Li, Y. A highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol. Catal. Sci. Technol. 2015, 5, 1169–1180. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, L.; Lv, J.; Wang, S.; Gong, J.; Ma, X. Hydrogenation of dimethyl oxalate to ethylene glycol on a Cu/SiO2/cordierite monolithic catalyst: Enhanced internal mass transfer and stability. AlChE J. 2012, 58, 2798–2809. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.-F.; Louis, C. Conditions of Formation of Copper Phyllosilicates in Silica-Supported Copper Catalysts Prepared by Selective Adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.-R.; Jaenicke, S.; Chuah, G.-K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Brew, D.; Glasser, F. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural characterization of magnesium silicate hydrate: Towards the design of eco-sustainable cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef]
- Pedone, A.; Palazzetti, F.; Barone, V. Models of Aged Magnesium–Silicate–Hydrate Cements Based on the Lizardite and Talc Crystals: A Periodic DFT-GIPAW Investigation. J. Phys. Chem. C 2017, 121, 7319–7330. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (M-S-H): The relation with 2:1 Mg–Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Walling, S.A.; Kinoshita, H.; Bernal, S.A.; Collier, N.C.; Provis, J.L. Structure and properties of binder gels formed in the system Mg(OH)2–SiO2–H2O for immobilisation of Magnox sludge. Dalton Trans. 2015, 44, 8126–8137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.; Whittaker, A.; Millar, G.; Zhu, H. Comprehensive study of surface chemistry of MCM-41 using 29Si CP/MAS NMR, FTIR, pyridine-TPD, and TGA. J. Phys. Chem. B 1997, 101, 6525–6531. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Murzin, D.Y. Effect of catalyst synthesis parameters on the metal particle size. Appl. Catal. A Gen. 2013, 451, 251–281. [Google Scholar] [CrossRef]
- Angelici, C.; Meirer, F.; van der Eerden, A.M.J.; Schaink, H.L.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J.M.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Ex Situ and Operando Studies on the Role of Copper in Cu-Promoted SiO2–MgO Catalysts for the Lebedev Ethanol-to-Butadiene Process. ACS Catal. 2015, 5, 6005–6015. [Google Scholar] [CrossRef]
- Cornu, D.; Lin, L.; Daou, M.M.; Jaber, M.; Krafft, J.-M.; Herledan, V.; Laugel, G.; Millot, Y.; Lauron-Pernot, H. Influence of acid–base properties of Mg-based catalysts on transesterification: Role of magnesium silicate hydrate formation. Catal. Sci. Technol. 2017, 7, 1701–1712. [Google Scholar] [CrossRef] [Green Version]
- Egloff, G.; Hulla, G. Conversion of Oxygen Derivatives of Hydrocarbons into Butadiene. Chem. Rev. 1945, 36, 63–141. [Google Scholar] [CrossRef]
- Natta, G.; Rigamonti, R. Fünfter Band: Heterogene Katalyse II; Springer: Vienna, Austria, 1957. [Google Scholar]
- Quattlebaum, W.M.; Toussaint, W.J.; Dunn, J.T. Deoxygenation of Certain Aldehydes and Ketones: Preparation of Butadiene and Styrene. J. Am. Chem. Soc. 1947, 69, 593–599. [Google Scholar] [CrossRef]
- Arundale, E.; Mikeska, L.A. The Olefin-Aldehyde Condensation. The Prins Reaction. Chem. Rev. 1952, 51, 505–555. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I. Ag-Promoted ZrBEA Zeolites Obtained by Post-Synthetic Modification for Conversion of Ethanol to Butadiene. Chemsuschem 2016, 9, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Pomalaza, G.; Capron, M.; Ordomsky, V.; Dumeignil, F. Recent Breakthroughs in the Conversion of Ethanol to Butadiene. Catalysts 2016, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Makshina, E.; Janssens, W.; Sels, B.; Jacobs, P.A. Catalytic study of the conversion of ethanol into 1,3-butadiene. Catal. Today 2012, 198, 338–344. [Google Scholar] [CrossRef]
- Li, S.; Men, Y.; Wang, J.; Liu, S.; Wang, X.; Ji, F.; Chai, S.; Song, Q. Morphological control of inverted MgO-SiO2 composite catalysts for efficient conversion of ethanol to 1, 3-butadiene. Appl. Catal. A 2019, 577, 1–9. [Google Scholar] [CrossRef]
- Chieregato, A.; Velasquez Ochoa, J.; Bandinelli, C.; Fornasari, G.; Cavani, F.; Mella, M. On the Chemistry of Ethanol on Basic Oxides: Revising Mechanisms and Intermediates in the Lebedev and Guerbet reactions. ChemSusChem 2015, 8, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Makshina, E.V.; Vanelderen, P.; De Clippel, F.; Houthoofd, K.; Kerkhofs, S.; Martens, J.A.; Jacobs, P.A.; Sels, B.F. Ternary Ag/MgO-SiO2 Catalysts for the Conversion of Ethanol into Butadiene. Chemsuschem 2015, 8, 994–1008. [Google Scholar] [CrossRef]
- Natta, G.; Rigamonti, R. Sintesi Del Butadiene Da Alcool Etilico; La Chimica e l’Industria: Milan, Italy, 1947; pp. 195–200. [Google Scholar]
- Yan, T.; Yang, L.; Dai, W.; Wang, C.; Wu, G.; Guan, N.; Hunger, M.; Li, L. On the deactivation mechanism of zeolite catalyst in ethanol to butadiene conversion. J. Catal. 2018, 367, 7–15. [Google Scholar] [CrossRef]
- Taifan, W.E.; Li, Y.; Baltrus, J.P.; Zhang, L.; Frenkel, A.I.; Baltrusaitis, J. Operando structure determination of Cu and Zn on supported MgO/SiO2 catalysts during ethanol conversion to 1, 3-butadiene. ACS Catal. 2018, 9, 269–285. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, Y.N.; Tan, X.; Wang, L.; Yu, Y.; Jiang, H. Study of ethanol/acetaldehyde to 1, 3-butadiene over MgO–SiO2 catalyst: Comparative investigation of deactivation behaviour due to carbon deposition. Catal. Lett. 2020, 150, 1462–1470. [Google Scholar] [CrossRef]
- Chae, H.-J.; Kim, T.-W.; Moon, Y.-K.; Kim, H.-K.; Jeong, K.-E.; Kim, C.-U.; Jeong, S.-Y. Butadiene production from bioethanol and acetaldehyde over tantalum oxide-supported ordered mesoporous silica catalysts. Appl. Catal. B Environ. 2013, 150–151, 596–604. [Google Scholar] [CrossRef]










| Sample | Surf. Area (m2/g) | Micropore Area (m2/g) | Pore Vol. (cm3/g) | Mg/Si a |
|---|---|---|---|---|
| MgO-SiO2-1 | 281 | 32 | 0.61 | 0.96 |
| MgO-SiO2-2 | 206 | 35 | 0.70 | 2.03 |
| MgO-SiO2-4 | 142 | 41 | 0.96 | 3.97 |
| MgO-SiO2-5 | 127 | 43 | 0.83 | 4.91 |
| MgO-SiO2-10 | 87 | 9 | 0.38 | 9.87 |
| MgO-SiO2-4 (WK) | 178 | 49 | 0.35 | 3.94 |
| MgO | 59 | 0 | 0.17 | - |
| SiO2 | 423 | 30 | 2.41 | - |
| Catalyst | T (°C) | Conv. (%) | BD Sel. (%) | BD Yield (%) | Productivity (gBD gcat−1 h−1) | Ref |
|---|---|---|---|---|---|---|
| MgO-SiO2-4 (AE) | 475 | 96 | 39 | 37 | 0.69 | This work |
| MgO-SiO2-4 (WK) | 475 | 94 | 16 | 15 | 0.27 | This work |
| MgO-SiO2 (WK) | 325 | 35 | 44 | 15 | 0.027 | [21] |
| MgO-SiO2 (WK) | 425 | 65 | 34 | 23 | 0.14 | [26] |
| MgO-SiO2 (WK) [a] | 450 | 67 | 63 | 42 | 1.00 | [60] |
| MgO-SiO2 (WK) [b] | 450 | 42 | 53 | 22 | 0.64 | [24] |
| MgO-SiO2 (Sol gel) | 400 | 40 | 40 | 16 | 0.22 | [19] |
| MgO-SiO2 (Co-precipitate) | 425 | 46 | 13 | 6 | - | [28] |
| MgO-SiO2 (Impregnation) [c] | 350 | 81 | 30 | 24 | 0.056 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsudin, I.B.; Jaenicke, S.; Chuah, G.-K. Conversion of Ethanol to Butadiene over Binary MgO-SiO2 Mixed Oxides Prepared by the Ammonia Evaporation Method. Chemistry 2023, 5, 544-558. https://doi.org/10.3390/chemistry5010039
Samsudin IB, Jaenicke S, Chuah G-K. Conversion of Ethanol to Butadiene over Binary MgO-SiO2 Mixed Oxides Prepared by the Ammonia Evaporation Method. Chemistry. 2023; 5(1):544-558. https://doi.org/10.3390/chemistry5010039
Chicago/Turabian StyleSamsudin, Ismail Bin, Stephan Jaenicke, and Gaik-Khuan Chuah. 2023. "Conversion of Ethanol to Butadiene over Binary MgO-SiO2 Mixed Oxides Prepared by the Ammonia Evaporation Method" Chemistry 5, no. 1: 544-558. https://doi.org/10.3390/chemistry5010039
APA StyleSamsudin, I. B., Jaenicke, S., & Chuah, G. -K. (2023). Conversion of Ethanol to Butadiene over Binary MgO-SiO2 Mixed Oxides Prepared by the Ammonia Evaporation Method. Chemistry, 5(1), 544-558. https://doi.org/10.3390/chemistry5010039