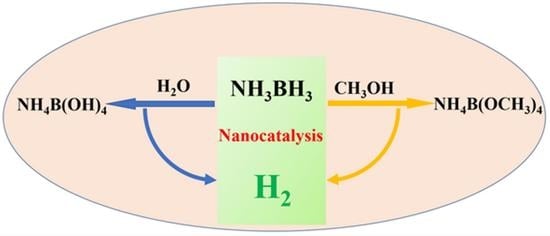
Hydrogen Evolution upon Ammonia Borane Solvolysis: Comparison between the Hydrolysis and Methanolysis Reactions
Abstract
:1. Introduction
2. Ammonia Borane (AB)
3. H2 Generation upon AB Hydrolysis
3.1. Use of an Acidic Medium
3.2. Use of a Transition-Metal Catalyst
3.3. Use of a Transition-Metal-Alloyed Catalyst
4. Catalyzed H2 Generation upon AB Methanolysis
5. Comparison between AB Hydrolysis and Methanolysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen Carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global Hydrogen Development—A Technical and Geopolitical Overview. Int. J. Hydrogen Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalyic Hydrogen Evolution. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Xu, Q.J. A High-Performance Hydrogen Generation System: Transition Metal-Catalyzed Dissociation and Hdrolysis of Ammonia-Borane. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B–N compounds for chemical hydrogenstorage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. B-N Compounds for Chemical Hydrogen Storage. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef]
- Wang, P. Molecular Systems for Ligh-Driven Hydrogen Production. Dalton Trans. 2012, 41, 4296e302. [Google Scholar]
- Zhan, W.-W.; Zhu, Q.-L.; Xu, Q. Dehydrogenation of Ammonia Borane by Metal Nanoparticle Catalysts. ACS Catal. 2016, 6, 6892–6905. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q. Metal-Nanocatalyzed Hydrogen Generation from Formic Acid. Acc. Chem. Res. 2017, 50, 1449–1458. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent Advances and Remaining Challenges of Nanosctuctured Materials for Hydrogen Storage Applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Demici, U.B. Ammonia borane, a material with exceptional properties for ammonia borane storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, R.; Xu, Q. Metal-Organic Framework Based Catalysts for Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801193. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia borane as hydrogen storage materials. Int. J. Hydrogen Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Sun, Q.M.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid Phase Chemical Hydrogen Storage. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.G.; Wang, S.N.; Dewhurst, R.D.; Ignat’ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its Role in Energy-Related Processes and Applications. Angew. Chem. Int. Ed. 2020, 59, 8800–8816. [Google Scholar] [CrossRef] [PubMed]
- Axet, M.R.; Philippot, K. Catalysis with Colloidal Ruthenium Nanoparticles. Chem. Rev. 2020, 120, 1085–1145. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.L.; Ding, Y.; Lu, Z.-H. Nobel-metal free catalysts for hydrogen generation from boron- and nitrogen-based hydrides. Inorg. Chem. Front. 2020, 7, 3837–3874. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Fu, F.; Astruc, D. Hydrogen Generation from Nanocatalyzed Hydrolysis of Hydrogen-Rich Boron Derivatives: Recent Developments. Acc. Chem. Res. 2020, 53, 2483–2493. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent Developments of Liquid-Phase Hydrogen Generation. Chem. Soc. Rev. 2021, 50, 3437–3484. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Lau, S.; Gasperini, D.; Webster, R.L. Ammonia Boranes as Transfer Hydrogenation and Hydrogenation Reagents. Angew. Chem. Int. Ed. 2021, 6, 14272–14294. [Google Scholar] [CrossRef] [PubMed]
- Mboyi, C.D.; Poinsot, D.; Roger, J.; Fajerwerg, K.; Kahn, M.L.; Hierso, J.-C. The Hydrogen-Storage Challenge: Nanoparticles for Metal-Catalyzed Ammonia Borane Dehydrgenation. Small 2021, 17, 2102759. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Li, D.-S.; Zhang, S.; Zhang, Q.J. Recent Advances in the “On-Off” Approaches for the on-Demand Liquid-Phase Hydrogen Evolution. Mater. Chem. A 2021, 9, 18164–18174. [Google Scholar] [CrossRef]
- Klooster, W.T.; Koetzle, T.F.; Siegbahn, P.E.M.; Richardson, T.B.; Crabtree, R.H. Study of the NH...H-B Dihydrogen Bond Including the Crystal Structure of BH3NH3 by Neutron Diffraction. J. Am. Chem. Soc. 1999, 121, 6337–6343. [Google Scholar] [CrossRef]
- Plumley, J.A.; Evanseck, J.D. Covalent and Ionic Nature of the Dative Bond and Account of Accurate Ammonia-Borane Binding Enthalpies. J. Phys. Chem. A 2007, 111, 13472–13483. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, R.W. The Cystalline Compound Ammonia Borane, H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Shore, S.G. Ammonia Borane, Past as Prolog. J. Organomet. Chem. 2014, 751, 60–66. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Raju, B.C.; Gagare, P.D. One-Pot Synthesis of Ammonia-Borane and Trialkylamine-Borane from Trimethyl Borate. Org. Lett. 2012, 14, 6119–6121. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Mayfield, D.L.; Gilbreath, J.R. The Preparation of Other Borohydrides by Metathetical Reactions Utilizing the Metal Alkali Borohydrides. J. Am. Chem. Soc. 1953, 75, 213–215. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Kulkarni, A.S. Open Flask Synthesis of Amine Boranes via Tandem Amine-Ammonium Salt Equilibration-Metathesis. Inorg. Chem. 2015, 54, 5618–5620. [Google Scholar] [CrossRef]
- Wiedner, E.S.; Chambers, M.B.; Pitman, C.L.; Bullock, R.M.; Miller, A.J.M.; Appel, A.M. Thermodynamic Hydricity of Transition Metal Hydrides. Chem. Rev. 2016, 116, 8655–8692. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Xu, Q.J. Dissociation and hydrolysis of ammonia-borane with solid acids and carbon dioxide: An efficient hydrogen generation system. Power Sources 2006, 159, 855–860. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Gagare, P.D. Preparation of Ammonia Borane in High Yield and Purity, Methanolysis, and Regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Krause, J.A.; Guan, H. Mechanistic Studies of Ammonia Borane Dehydrogenation Catalyzed by Iron Pincer Complexes. J. Am. Chem. Soc. 2014, 136, 11153–11161. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Transition-metal radicals: Chameleon structure and catalytic function. Acc. Chem. Res. 1991, 24, 36–42. [Google Scholar] [CrossRef]
- Buss, J.A.; Edouard, G.A.; Cheng, C.; Shi, J.; Agapie, T. Molybdenum Catalyzed Ammonia Borane Dehydrogenation. J. Am. Chem. Soc. 2014, 136, 11272–11275. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Li, D.; Wang, Z.; Qian, G.; Sui, Z.; Duan, X.; Zhou, X.; Yeboah, I.; Chen, D. Reaction mechanism and kinetics for hydrolytic dehydrogenation of ammonia borane on a Pt/CNT catalyst. AIChE J. 2017, 63, 60–65. [Google Scholar] [CrossRef]
- Li, Z.; He, T.; Liu, L.; Chen, W.; Zhang, M.; Wu, G.; Chen, P. Covalent triazine framework supported non-noble metal nanoparticles with superior activity for catalytic hydrolysis of ammonia borane: From mechanistic study to catalyst design. Chem. Sci. 2017, 8, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Statakis, M.; Garcia, H. Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Cha, D.; Zhang, X.X.; Basset, J.M. High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Figri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar]
- Narayanan, R.; El-Sayed, B.J. Catalysis with Transition Metal Nanoparticles in Colloidal Solution: Nanoparticle Shape Dependence and Stability. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar] [CrossRef] [PubMed]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Li, G.; Jin, R.C. Atomically Precise Gold Nanoclusters as New Model Catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Li, Y.D. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Sboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Astruc, D.; Heuze, K.; Gatard, S.; Méry, D.; Nlate, S.; Plault, L. Metallodendritic Catalysis for Redox and Carbon—Carbon Bond Formation Reactions: A Step towards Green Chemistry. Adv. Syn. Catal. 2005, 347, 329–338. [Google Scholar] [CrossRef]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corma, A.; Garcia, H.; Xamena, F.X.L. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Viciano-Chumillas, M.; Mon, M.; Ferrando-Soria, J.; Corma, A.; Leyva-Perez, A.; Armentano, D.; Pardo, E. Metal–Organic Frameworks as Chemical Nanoreactors: Synthesis and Stabilization of Catalytically Active Metal Species in Confined Spaces. Acc. Chem. Res. 2020, 53, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Na, K.; Somorjai, G.A.; Yaghi, O.M. Chemical Environment Control and Enhanced Catalytic Performance of Platinum Nanoparticles Embedded in Nanocrystalline Metal–Organic Frameworks. J. Am. Chem. Soc. 2015, 137, 7810–7816. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Li, Z.H.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, M.; Garcia, H. Metal–Organic Frameworks as Multifunctional Solid Catalysts. Trends Chem. 2020, 2, 454–466. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974–986. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.D.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Pei, X.; Kalmutzki, M.J.; Yang, J.; Yaghi, O.M. Large Cages of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2022, 55, 707–721. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Yaghi, O.M.; Somorjai, G.A. Metal Nanocrystals Embedded in Single Nanocrystals of MOFs Give Unusual Selectivity as Heterogeneous Catalysts. Nano Lett. 2014, 14, 5979–5983. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper Nanocrystals Encapsulated in Zr-based Metal–Organic Frameworks for Highly Selective CO2 Hydrogenation to Methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef] [PubMed]
- Michaud, P.; Astruc, D.; Ammeter, J.H. Electron-transfer pathways in the reduction of d6 and d7 organoiron cations by lithium tetrahydroaluminate and sodium tetrahydroborate. J. Am. Chem. Soc. 1982, 104, 3755–3757. [Google Scholar] [CrossRef]
- Wang, C.; Tuminetti, J.; Wang, Z.; Zhang, C.; Ciganda, R.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of Ammonia-Borane over Ni/ZIF-8 Nanocatalyst: High Efficiency, Mechanism, and Controlled Hydrogen Release. J. Am. Chem. Soc. 2017, 139, 11610–11615. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Chong, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E.; et al. Highly Selective and Sharp Volcano-type Synergistic Ni2Pt@ZIF-8-Catalyzed Hydrogen Evolution from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Wu, Y.; Astruc, D. PtNi@ZIF-8 nanocatalyzed high efficiency and complete hydrogen generation from hydrazine borane: Origin and mechanistic insight. J. Mater. Chem. A 2022, 10, 17614–17623. [Google Scholar] [CrossRef]
- Kang, N.; Wei, X.; Shen, R.; Li, B.; Guisasola, E.; Cal, E.; Moya, S.; Salmon, L.; Wang, C.; Coy, E.; et al. Fast Au-Ni@ZIF-8-catalyzed ammonia borane hydrolysis boosted by dramatic volcano-type synergy and plasmonic acceleration. Appl. Catal. B 2023, 320, 121957. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Astruc, D. Tetrahydroxydiboron (bis-boric acid), a versatile reagent for borylation, hydrogenation, catalysis, radical reactions and H2 generation. Eur. J. Inorg. Chem. 2023. [Google Scholar] [CrossRef]
- Hou, C.-C.; Li, Q.; Wang, C.-J.; Peng, C.-Y.; Chen, Q.-Q.; Ye, H.-F.; Fu, W.-F.; Che, C.-M.; López, N.; Chen, Y. Ternary Ni–Co–P nanoparticles as noble-metal-free catalysts to boost the hydrolytic dehydrogenation of ammonia-borane. Energy Environ. Sci. 2017, 10, 1770–1776. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Hao, Y.; Wang, C.; Zhang, X.; Yu, X.; Yang, X.; Li, L.; Lu, Z.; Shang, W. TiO2–CdS supported CuNi nanoparticles as a highly efficient catalyst for hydrolysis of ammonia borane under visible-light irradiation. Int. J. Hydrogen Energy 2020, 45, 4390–4402. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, F.; Yang, S.; Martinez Moro, M.; Ramirez, M.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. Dramatic Synergy in CoPt Nanocatalysts Stabilized by “Click” Dendrimers for Evolution of Hydrogen from Hydrolysis of Ammonia Borane. ACS Catal. 2019, 9, 1110–1119. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [Green Version]
- Gellé, A.; Jin, T.; de la Garza, L.; Price, G.D.; Besteiro, L.V.; Moores, A. Applications of Plasmon-Enhanced Nanocatalysis to Organic Transformations. Chem. Rev. 2020, 120, 986–1041. [Google Scholar] [CrossRef] [PubMed]
- Zada, A.; Muhammad, P.; Ahmad, W.; Hussain, Z.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Surface Plasmonic-Assisted Photocatalysis and Optoelectronic Devices with Noble Metal Nanocrystals: Design, Synthesis, and Applications. Adv. Funct. Mater. 2019, 30, 1906744. [Google Scholar] [CrossRef]
- Wen, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of Catalytic Activity Over AuPd Nanoparticles Loaded Metal Organic Framework Under Visible Light Irradiation. Top. Catal. 2016, 59, 1765–1771. [Google Scholar] [CrossRef]
- Rej, S.; Hsia, C.-F.; Chen, T.-Y.; Lin, F.-C.; Huang, J.-S.; Huang, M.H. Facet-Dependent and Light-Assisted Efficient Hydrogen Evolution from Ammonia Borane Using Gold–Palladium Core–Shell Nanocatalysts. Angew. Chem. Int. Ed. 2016, 55, 7222–7226. [Google Scholar] [CrossRef]
- Verma, P.; Yuan, K.; Kuwahara, Y.; Mori, K.; Yamashita, H. Enhancement of plasmonic activity by Pt/Ag bimetallic nanocatalyst supported on mesoporous silica in the hydrogen production from hydrogen storage material. Appl. Catal. B 2018, 223, 10–15. [Google Scholar] [CrossRef]
- Kang, N.; Wang, Q.; Djeda, R.; Wang, W.; Fu, F.; Martinez Moro, M.; Ramirez, M.A.S.; Moya, E.; Coy, L.; Salmon, J.-L.; et al. Visible-Light Acceleration of H2 Evolution from Aqueous Solutions of Inorganic Hydrides Catalyzed by Gold-Transition-Metal Nanoalloys. ACS Appl. Mater. Interfaces 2020, 12, 53816–53826. [Google Scholar] [CrossRef]
- Zhang, Y.C.; He, S.; Guo, W.X.; Hu, Y.; Huang, J.W.; Mulcahy, J.R.; Wei, D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Gong, J.L. Mechanistic Understanding of the Plasmonic Enhancement for Solar Water Splitting. Adv. Mater. 2015, 27, 5328–5342. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Sanyal, U.; Jagirdar, B.R. Nanostructured Cu and Cu@Cu2O core shell catalysts for hydrogen generation from ammonia–borane. Phys. Chem. Chem. Phys. 2008, 10, 5870–5874. [Google Scholar] [CrossRef] [Green Version]
- Kalidindi, S.B.; Vernekar, A.A.; Jagirdar, B.R. Co–Co2B, Ni–Ni3B and Co–Ni–B nanocomposites catalyzed ammonia–borane methanolysis for hydrogen generation. Phys. Chem. Chem. Phys. 2009, 11, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.H.; Li, P.Y.; Xu, Y.; Huang, J.L.; Li, Q. Monodisperse AgPd alloy nanoparticles as a highly active catalyst towards the methanolysis of ammonia borane for hydrogen generation. RSC Adv. 2016, 6, 105940–105947. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, C.; Luo, M.; Yao, Q.; Lu, Z.-H. Ultrafine Rh nanoparticles confined by nitrogen-rich covalent organic frameworks for methanolysis of ammonia borane. Inorg. Chem. Front. 2020, 7, 1298–1306. [Google Scholar] [CrossRef]
- Li, X.; Yao, Q.; Li, Z.; Li, H.; Zhu, Q.-L.; Lu, Z.-H. Modulation of Cu and Rh single-atoms and nanoparticles for high-performance hydrogen evolution activity in acidic media. J. Mater. Chem. A 2021, 10, 326–336. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, W.; Hu, M.; Wang, Q.; Cheng, X.; Zhang, Y.; Wang, Y.; Gao, D.; Bi, J.; Fan, G. Ultrahigh Catalytic Activity of l-Proline-Functionalized Rh Nanoparticles for Methanolysis of Ammonia Borane. ChemSusChem 2019, 12, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Caner, N.; Yurderi, M.; Bulut, A.; Kanberoglu, G.S.; Kaya, M.; Zahmakiran, M. Chromium based metal-organic framework MIL-101 decorated palladium nanoparticles for the methanolysis of ammonia-borane. New J. Chem. 2020, 44, 12435–12439. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.; Wang, X.; Zhu, Y.; Chen, Y. Review on Hydrogen Production from Catalytic Ammonia Borane Methanolysis: Advances and Perspectives. Energy Fuels 2022, 36, 11745–11759. [Google Scholar] [CrossRef]
- Özkar, S. Transition metal nanoparticle catalysts in releasing hydrogen from the methanolysis of ammonia borane. Int. J. Hydrogen Energy 2020, 45, 7881–7891. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Ertas, İ.E.; Zahmakiran, M.; Kaya, M. Supported copper–copper oxide nanoparticles as active, stable and low-cost catalyst in the methanolysis of ammonia–borane for chemical hydrogen storage. Appl. Catal. B 2015, 165, 169–175. [Google Scholar] [CrossRef]
- Tunc, N.; Rakap, M. Preparation and characterization of Ni-M (M: Ru, Rh, Pd) nanoclusters as efficient catalysts for hydrogen evolution from ammonia borane methanolysis. Renew. Energy 2020, 155, 1222–1230. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, L.; Dushimimana, F.; Zhao, J.; Wang, S.; Han, J.; Zhu, X.; Liu, X.; Ge, Q.F.; et al. Visible-Light-Driven Multichannel Regulation of Local Electron Density to Accelerate Activation of O-H and B-H Bonds for Ammonia Borane Hydrolysis. ACS Catal. 2020, 10, 14903–14915. [Google Scholar] [CrossRef]
- Kang, N.; Shen, R.; Li, B.; Fu, F.; Espuche, B.; Moya, S.; Salmon, L.; Pozzo, J.-L.; Astruc, D. Dramatic acceleration by visible light and mechanism of AuPd@ZIF-8-catalyzed ammonia borane methanolysis for efficient hydrogen production. J. Mater. Chem. A 2023, 11, 5245–5256. [Google Scholar] [CrossRef]
- Jo, S.; Verma, P.; Kuwahara, Y.; Mori, K.; Choi, W.; Yamashita, H. Enhanced hydrogen production from ammonia borane using controlled plasmonic performance of Au nanoparticles deposited on TiO2. J. Mater. Chem. A 2017, 5, 21883–21892. [Google Scholar] [CrossRef]
- Ashby, C.; Dobbs, F.R.; Harry Hopkins, P.J., Jr. Composition of lithium aluminum hydride, lithium borohydride, and their alkoxy derivatives in ether solvents as determined by molecular association and conductance studies. J. Am. Chem. Soc. 1975, 97, 3158–3162. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.Q.; Qiao, B.T.; Li, J.; Liu, J.Y.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Liu, L.C.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Qin, F.; Wang, S.; Wu, J.; Li, R.; Wang, Z.; Ren, M.; Liu, D.; Wang, D.; Astruc, D. Overview of the materials design and sensing strategies of nanopore devices. Coord. Chem. Rev. 2023, 478, 214998. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Ouyang, J.; Chen, K.; Zhu, M.; Liu, Z.W. Hydrogen Production via Hydrolysis and Alcoholysis of Light Metal-Based Materials: A Review. Nano-Micro Lett. 2021, 13, 134. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.H.; Shao, Z.P.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.; Wang, C.; Astruc, D. Hydrogen Evolution upon Ammonia Borane Solvolysis: Comparison between the Hydrolysis and Methanolysis Reactions. Chemistry 2023, 5, 886-899. https://doi.org/10.3390/chemistry5020060
Kang N, Wang C, Astruc D. Hydrogen Evolution upon Ammonia Borane Solvolysis: Comparison between the Hydrolysis and Methanolysis Reactions. Chemistry. 2023; 5(2):886-899. https://doi.org/10.3390/chemistry5020060
Chicago/Turabian StyleKang, Naixin, Changlong Wang, and Didier Astruc. 2023. "Hydrogen Evolution upon Ammonia Borane Solvolysis: Comparison between the Hydrolysis and Methanolysis Reactions" Chemistry 5, no. 2: 886-899. https://doi.org/10.3390/chemistry5020060
APA StyleKang, N., Wang, C., & Astruc, D. (2023). Hydrogen Evolution upon Ammonia Borane Solvolysis: Comparison between the Hydrolysis and Methanolysis Reactions. Chemistry, 5(2), 886-899. https://doi.org/10.3390/chemistry5020060