A Missing Nuclearity in the Co(III)/Ln(III)/2-Pyridyladoxime Chemistry: Tetranuclear Compounds Using the “Assisted Self-Assembly” Approach (Ln = Rare Earth Metals) †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Spectroscopic–Physical Measurements
2.2. Preparation of the Complexes
2.3. Single-Crystal X-ray Crystallography
3. Results and Discussion
3.1. Synthetic Comments
3.2. Characterization of the Products
3.2.1. Vibrational Spectroscopy
3.2.2. Energy-Dispersive X-ray (EDX) and Powder X-ray Diffraction (PXRD) Analysis of the Complexes
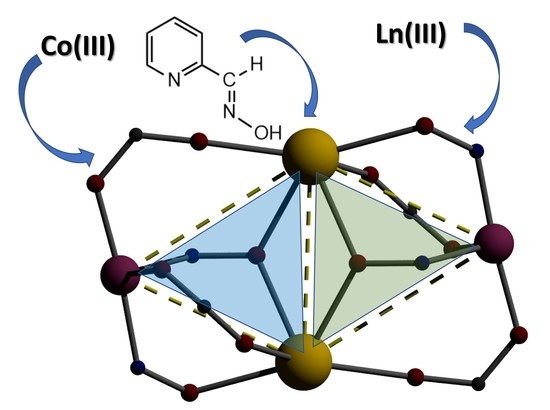
3.3. Description of the Structure
4. Concluding Comments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- James, C.; Willand, P.S. The rare earth cobalticyanide. J. Am. Chem. Soc. 1916, 38, 1497–1500. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-S.; Zhang, K.; Song, Y.; Pan, Z.-Q. Recent advances in 3d-4f magnetic complexes with several types of non-carboxylate organic ligands. Inorg. Chim. Acta 2021, 521, 120318. [Google Scholar] [CrossRef]
- Sharples, J.W.; Collison, D. The coordination chemistry and magnetism of some 3d–4f and 4f amino-polyalcohol compounds. Coord. Chem. Rev. 2014, 260, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lada, Z.G.; Polyzou, C.D.; Nika, V.; Stamatatos, T.C.; Konidaris, K.F.; Perlepes, S.P. Adventures in the coordination chemistry of 2-pyridyl oximes: On the way to 3d/4f-metal coordination clusters. Inorg. Chim. Acta 2022, 539, 120954. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Evangelisti, M.; Winpenny, R.E.P. Co-Gd phosphonate complexes as magnetic refrigerants. Chem. Sci. 2011, 2, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Boulkedid, A.-L.; Long, J.; Beghidja, C.; Guari, Y.; Beghidja, A.; Larionova, J. A luminescent Schiff-base heterotrinuclear Zn2Dy single-molecule magnet with an axial crystal field. Dalton Trans. 2018, 47, 1402–1406. [Google Scholar] [CrossRef]
- Nikolaevskii, S.A.; Petrov, P.A.; Sukhikh, T.S.; Yambulatov, D.S.; Kiskin, M.A.; Sokolov, M.N.; Eremenko, I.L. Simple synthetic protocol to obtain 3d-4f-heterometallic carboxylate complexes of N-heterocyclic carbenes. Inorg. Chim. Acta 2020, 508, 119643. [Google Scholar] [CrossRef]
- Nikolaevskii, S.A.; Yambulatov, D.S.; Voronina, J.K.; Melnikov, S.N.; Babeshkin, K.A.; Efimov, N.N.; Goloveshkin, A.S.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. The First Example of 3 d-4 f-Heterometallic Carboxylate Complex Containing Phosphine Ligand. Chem. Sel. 2020, 5, 12829–12834. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Yambulatov, D.S.; Nikolaevskii, S.A.; Shmelev, M.A.; Babeshkin, K.A.; Efimov, N.N.; Poddel’sky, A.I.; Eremenko, I.L.; Kiskin, M.A. The First Tetranuclear Iron(II)-Gadolinium(III) Carboxylate Complex [Fe2Gd2(piv)10(bpy)2]: Synthesis, Structure Elucidation and Magnetic Properties. Chem. Sel. 2022, 7, e202203612. [Google Scholar]
- Chorazy, S.; Zychowicz, M.; Ohkoshi, S.-I.; Sieklucka, B. Wide-Range UV-to-Visible Excitation of Near-Infrared Emission and Slow Magnetic Relaxation in LnIII(4,4′-Azopyridine-1,1′-dioxide)[CoIII(CN)6]3– Layered Frameworks. Inorg. Chem. 2019, 58, 165–179. [Google Scholar] [CrossRef]
- Andruh, M.; Costes, J.-P.; Diaz, C.; Gao, S. 3d−4f Combined Chemistry: Synthetic Strategies and Magnetic Properties. Inorg. Chem. 2009, 48, 3342–3359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zakrzewski, J.J.; Heczko, M.; Zychowicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S.-I. Proton Conductive Luminescent Thermometer Based on Near-Infrared Emissive {YbCo2} Molecular Nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Alexandru, M.-G.; Visinescu, D.; Shova, S.; Oliveira, W.X.C.; Lloret, F.; Julve, M. Design of 3d–4f molecular squares through the [Fe{(HB(pz)3)}(CN)3]− metalloligand. Dalton Trans. 2018, 47, 6005–6017. [Google Scholar] [CrossRef]
- Sessoli, R.; Powell, A.K. Strategies towards single-molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Qin, T.; Shi, Z.; Fu, P.; Xiong, D.; Dong, X. A comparative study of proton conduction between two new Cd(II) and Co(II) complexes and in vitro antibacterial study of the Cd(II) complex. Appl. Organomet. Chem. 2023, 37, e6920. [Google Scholar] [CrossRef]
- Qin, T.; Shi, Z.; Zhang, W.; Dong, X.; An, N.; Sakiyama, H.; Muddassir, M.; Srivastava, D.; Kumar, A. 2D isostructural Ln(III)-based coordination polymer derived from Imidazole carboxylic acid: Synthesis, structure and magnetic behavior. J. Mol. Struct. 2023, 1282, 135220. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Z.; Li, D.; Li, Y.; An, N.; Shang, Y.; Sakiyama, H.; Muddassir, M.; Si, C. The regulation research of topology and magnetic exchange models of CPs through Co(II) concentration adjustment. J. Solid State Chem. 2023, 318, 123713. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Xu, M.; Xiong, C.; Hong, D.; Fang, H.; Cui, P. Dinitrogen Complexes of Cobalt(−I) Supported by Rare-Earth Metal-Based Metalloligands. Inorg. Chem. 2023, 62, 3836–3846. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 289, 74–122. [Google Scholar] [CrossRef]
- Polyzou, C.D.; Koumousi, E.S.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Rouzières, M.; Tsipis, A.C.; Mathonière, C.; Clérac, R.; Perlepes, S.P. “Switching on” the single-molecule magnet properties within a series of dinuclear cobalt(iii)–dysprosium(iii) 2-pyridyloximate complexes. Dalton Trans. 2017, 46, 14812–14825. [Google Scholar] [CrossRef]
- Anastasiadis, N.C.; Lada, Z.G.; Polyzou, C.D.; Raptopoulou, C.P.; Psycharis, V.; Konidaris, K.F.; Perlepes, S.P. Synthetic strategies to {CoIII2LnIII} complexes based on 2-pyridyl oximes (Ln = lanthanide). Inorg. Chem. Commun. 2019, 108, 107478. [Google Scholar] [CrossRef]
- Aromí, G.; Batsanov, A.S.; Christian, P.; Helliwell, M.; Parkin, A.; Parsons, S.; Smith, A.A.; Timco, G.A.; Winpenny, R.E.P. Synthetic and Structural Studies of Cobalt–Pivalate Complexes. Chem. A Eur. J. 2003, 9, 5142–5161. [Google Scholar] [CrossRef] [PubMed]
- Rigaku MSC CrystalClear; Rigaku MSC Inc.: The Woodlands, TX, USA, 2005.
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- DIAMOND—Crystal and Molecular Structure Visualization, version 3.1; Crystal Impact: Bonn, Germany, 2014.
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; pp. 254–257. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxyalte coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- SHAPE, version 20; Universitat de Barcelona: Barcelona, Spain, 2010.
- Palenik, G.J. Bond Valence Sums in Coordination Chemistry Using Oxidation State Independent R0 Values. Inorg. Chem. 1997, 36, 122. [Google Scholar] [CrossRef]





| Formula | C48H62Co2Dy2N14O24 |
|---|---|
| F.W. | 1661.97 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 11.1135(2) |
| b (Å) | 11.7919(2) |
| c (Å) | 13.7354(3) |
| α (°) | 72.622(1) |
| β (°) | 81.670(1) |
| γ (°) | 67.216(1) |
| V (Å3) | 1582.94(5) |
| Z | 1 |
| T (°C) | −113 |
| Radiation | Cu Kα |
| ρcalcd (g cm−3) | 1.743 |
| μ (mm−1) | 17.201 |
| Reflections with I > 2σ(I) | 3305 |
| R1 a | 0.0964 |
| wR2 a | 0.2183 |
| Interatomic Distance (Å) | Interatomic Distance (Å) | ||
|---|---|---|---|
| Co-N(3) | 1.865(13) | Co-N(1) | 1.869(11) |
| Co-O(5) | 1.898(10) | Co-O(4) | 1.899(9) |
| Co-N(4) | 1.930(11) | Co-N(2) | 1.944(11) |
| Dy-O(3′) | 2.240(11) | Dy-O(6) | 2.311(8) |
| Dy-O(1) | 2.370(8) | Dy-O(1′) | 2.386(8) |
| Dy-O(2′) | 2.403(8) | Dy-O(10) | 2.408(9) |
| Dy-O(7) | 2.465(9) | Dy-O(8) | 2.497(9) |
| Dy-O(12) | 2.549(9) | Co…Dy | 4.466(3) |
| Co…Dy’ | 4.053(2) | Dy…Dy’ | 4.106(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lada, Z.G.; Katsoulakou, E.; Polyzou, C.D.; Raptopoulou, C.P.; Psycharis, V. A Missing Nuclearity in the Co(III)/Ln(III)/2-Pyridyladoxime Chemistry: Tetranuclear Compounds Using the “Assisted Self-Assembly” Approach (Ln = Rare Earth Metals). Chemistry 2023, 5, 996-1005. https://doi.org/10.3390/chemistry5020068
Lada ZG, Katsoulakou E, Polyzou CD, Raptopoulou CP, Psycharis V. A Missing Nuclearity in the Co(III)/Ln(III)/2-Pyridyladoxime Chemistry: Tetranuclear Compounds Using the “Assisted Self-Assembly” Approach (Ln = Rare Earth Metals). Chemistry. 2023; 5(2):996-1005. https://doi.org/10.3390/chemistry5020068
Chicago/Turabian StyleLada, Zoi G., Eugenia Katsoulakou, Christina D. Polyzou, Catherine P. Raptopoulou, and Vassilis Psycharis. 2023. "A Missing Nuclearity in the Co(III)/Ln(III)/2-Pyridyladoxime Chemistry: Tetranuclear Compounds Using the “Assisted Self-Assembly” Approach (Ln = Rare Earth Metals)" Chemistry 5, no. 2: 996-1005. https://doi.org/10.3390/chemistry5020068
APA StyleLada, Z. G., Katsoulakou, E., Polyzou, C. D., Raptopoulou, C. P., & Psycharis, V. (2023). A Missing Nuclearity in the Co(III)/Ln(III)/2-Pyridyladoxime Chemistry: Tetranuclear Compounds Using the “Assisted Self-Assembly” Approach (Ln = Rare Earth Metals). Chemistry, 5(2), 996-1005. https://doi.org/10.3390/chemistry5020068