Probing Low-Temperature OCM Performance over a Dual-Domain Catalyst Bed
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Catalyst Preparation
2.2. Characterization Methods
2.3. Catalytic Testing
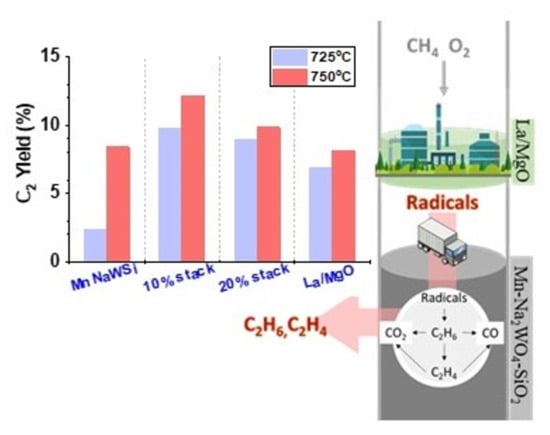
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, G.E.; Bhasin, M.M. Synthesis of Ethylene via Oxidative Coupling of Methane. I. Determination of Active Catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Revisiting the Oxidative Coupling of Methane to Ethylene in the Golden Period of Shale Gas: A Review. J. Ind. Eng. Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. In Applied Microbiology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 849–860. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Baltrusaitis, J.; Wachs, I.E. Oxidative Coupling of Methane (OCM) by SiO2-Supported Tungsten Oxide Catalysts Promoted with Mn and Na. ACS Catal. 2019, 9, 5912–5928. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn-Na2WO4/SiO2 as Catalyst for the Oxidative Coupling of Methane. What Is Really Known? Appl. Catal. A Gen. 2012, 425–426, 53–61. [Google Scholar] [CrossRef]
- Xueping, F.; Shuben, L.; Jingzhi, L.; Yanlai, C. Oxidative Coupling of Methane on W-Mn Catalysts. J. Mol. Catal. 1992, 6, 427–433. [Google Scholar]
- Kiani, D.; Sourav, S.; Wachs, I.E.; Baltrusaitis, J. Synthesis and Molecular Structure of Model Silica-Supported Tungsten Oxide Catalysts for Oxidative Coupling of Methane (OCM). Catal. Sci. Technol. 2020, 10, 3334–3345. [Google Scholar] [CrossRef]
- Li, S.B. Oxidative Coupling of Methane over W-Mn/SiO2 Catalyst. Chin. J. Chem. 2001, 19, 16–21. [Google Scholar] [CrossRef]
- Wang, D.J.; Rosynek, M.P.; Lunsford, J.H. Oxidative Coupling of Methane over Oxide-Supported Sodium-Manganese Catalysts. J. Catal. 1995, 155, 390–402. [Google Scholar] [CrossRef]
- Werny, M.J.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Fluctuating Storage of the Active Phase in a Mn-Na2WO4/SiO2 Catalyst for the Oxidative Coupling of Methane. Angew. Chem. 2020, 132, 15031–15036. [Google Scholar] [CrossRef]
- Sinev, M.; Ponomareva, E.; Sinev, I.; Lomonosov, V.; Gordienko, Y.; Fattakhova, Z.; Shashkin, D. Oxygen Pathways in Oxidative Coupling of Methane and Related Processes. Case Study: NaWMn/SiO2 Catalyst. Catal. Today 2019, 333, 36–46. [Google Scholar] [CrossRef]
- Hayek, N.S.; Lucas, N.S.; Warwar Damouny, C.; Gazit, O.M. Critical Surface Parameters for the Oxidative Coupling of Methane over the Mn-Na-W/SiO2 Catalyst. ACS Appl. Mater. Interfaces 2017, 9, 40404–40411. [Google Scholar] [CrossRef]
- Huang, B.; Hayek, N.S.; Sun, G.; Mottaghi-Tabar, S.; Simakov, D.S.A.; Gazit, O.M. Correlating Properties of the Mn2O3-Na2WO4/SiO2 Catalyst with Statistically Estimated Parameters for the Oxidative Coupling of Methane. Energy Fuels 2021, 35, 9589–9598. [Google Scholar] [CrossRef]
- Pak, S.; Qiu, P.; Lunsford, J.H. Elementary Reactions in the Oxidative Coupling of Methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO Catalysts. J. Catal. 1998, 179, 222–230. [Google Scholar] [CrossRef]
- Hayek, N.S.; Khlief, G.J.; Horani, F.; Gazit, O.M. Effect of Reaction Conditions on the Oxidative Coupling of Methane over Doped MnOx-Na2WO4/SiO2 Catalyst. J. Catal. 2019, 376, 25–31. [Google Scholar] [CrossRef]
- Lomonosov, V.I.; Sinev, M.Y. Oxidative Coupling of Methane: Mechanism and Kinetics. Kinet. Catal. 2016, 57, 647–676. [Google Scholar] [CrossRef]
- Martin, G.A.; Mirodatos, C. Surface Chemistry in the Oxidative Coupling of Methane. Fuel Process. Technol. 1995, 42, 179–215. [Google Scholar] [CrossRef]
- Horn, R.; Schlögl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.D.; Lunsford, J.H. Contribution of Gas-Phase Radical Coupling in the Catalytic Oxidation of Methane. J. Phys. Chem. 1988, 92, 5792–5796. [Google Scholar] [CrossRef]
- Tong, Y.; Rosynek, M.P.; Lunsford, J.H. Secondary Reactions of Methyl Radicals with Lanthanide Oxides: Their Role in the Selective Oxidation of Methane. J. Phys. Chem. 1989, 93, 2896–2898. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Z.Q.; Li, Z.; Wang, J.; Xu, M.; Zou, S.; Yang, J.; Pan, Y.; Gong, X.Q.; Xiao, L.; et al. CH3•-Generating Capability as a Reactivity Descriptor for Metal Oxides in Oxidative Coupling of Methane. ACS Catal. 2021, 11, 14651–14659. [Google Scholar] [CrossRef]
- Lin, C.H.; Campbell, K.D.; Wang, J.X.; Lunsford, J.H. Oxidative Dimerization of Methane over Lanthanum Oxide. J. Phys. Chem. 1986, 90, 534–537. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Qin, S.; Hu, C. La-Promoted Na2WO4/Mn/SiO2 Catalysts for the Oxidative Conversion of Methane Simultaneously to Ethylene and Carbon Monoxide. Appl. Catal. A Gen. 2007, 323, 126–134. [Google Scholar] [CrossRef]
- Ismagilov, I.Z.; Matus, E.V.; Vasil’Ev, S.D.; Kuznetsov, V.V.; Kerzhentsev, M.A.; Ismagilov, Z.R. Oxidative Condensation of Methane in the Presence of Modified MnNaW/SiO2 Catalysts. Kinet. Catal. 2015, 56, 456–465. [Google Scholar] [CrossRef]
- Ghose, R.; Hwang, H.T.; Varma, A. Oxidative Coupling of Methane Using Catalysts Synthesized by Solution Combustion Method: Catalyst Optimization and Kinetic Studies. Appl. Catal. A Gen. 2014, 472, 39–46. [Google Scholar] [CrossRef]
- Zhao, M.; Ke, S.; Wu, H.; Xia, W.; Wan, H. Flower-like Sr-La2O3 Microspheres with Hierarchically Porous Structures for Oxidative Coupling of Methane. Ind. Eng. Chem. Res. 2019, 58, 22847–22856. [Google Scholar] [CrossRef]
- Hou, Y.H.; Han, W.C.; Xia, W.S.; Wan, H.L. Structure Sensitivity of La2O2CO3 Catalysts in the Oxidative Coupling of Methane. ACS Catal. 2015, 5, 1663–1674. [Google Scholar] [CrossRef]
- Noon, D.; Seubsai, A.; Senkan, S. Oxidative Coupling of Methane by Nanofiber Catalysts. ChemCatChem 2013, 5, 146–149. [Google Scholar] [CrossRef]
- Song, J.; Sun, Y.; Ba, R.; Huang, S.; Zhao, Y.; Zhang, J.; Sun, Y.; Zhu, Y. Monodisperse Sr–La2O3 Hybrid Nanofibers for Oxidative Coupling of Methane to Synthesize C2 Hydrocarbons. Nanoscale 2015, 7, 2260–2264. [Google Scholar] [CrossRef]
- Jaroenpanon, K.; Tiyatha, W.; Chukeaw, T.; Sringam, S.; Witoon, T.; Wattanakit, C.; Chareonpanich, M.; Faungnawakij, K.; Seubsai, A. Synthesis of Na2WO4-MnxOy Supported on SiO2 or La2O3 as Fiber Catalysts by Electrospinning for Oxidative Coupling of Methane: Synthesis of Na2WO4-MnxOy Supported on SiO2 or La2O3 as Fiber Catalysts. Arab. J. Chem. 2022, 15, 103577. [Google Scholar] [CrossRef]
- Zou, S.; Li, Z.; Zhou, Q.; Pan, Y.; Yuan, W.; He, L.; Wang, S.; Wen, W.; Liu, J.; Wang, Y.; et al. Surface Coupling of Methyl Radicals for Efficient Low-Temperature Oxidative Coupling of Methane. Chin. J. Catal. 2021, 42, 1117–1125. [Google Scholar] [CrossRef]
- Rollins, B.; Grosjean, N.; Poulston, S. The Impact of the Presence of Various Relevant Gases during Aging of MnNa2WO4/SiO2 Catalyst on Its Performance for the Oxidative Coupling of Methane. Appl. Catal. A Gen. 2020, 598, 117606. [Google Scholar] [CrossRef]
- Hayek, N.S.; Lucas, N.S.; Warwar Damouny, C.; Gazit, O.M. A Comparative Study of Precursor Effect on Manganese Post-Synthetic Incorporation into the T-Sites of Dealuminated B-Zeolite. Microporous Mesoporous Mater. 2017, 244, 31–36. [Google Scholar] [CrossRef]
- Haibel, E.; Berendts, S.; Walter, D. Thermogravimetric and X-ray Diffraction Investigation on Carbonated Lanthanum Oxide and Lanthanum Hydroxide Formed in Humid CO2 Atmosphere. J. Therm. Anal. Calorim. 2018, 134, 261–267. [Google Scholar] [CrossRef]
- Sadjadi, S.; Jašo, S.; Godini, H.R.; Arndt, S.; Wollgarten, M.; Blume, R.; Görke, O.; Schomäcker, R.; Wozny, G.; Simon, U. Feasibility Study of the Mn–Na2WO4/SiO2 Catalytic System for the Oxidative Coupling of Methane in a Fluidized-Bed Reactor. Catal. Sci. Technol. 2015, 5, 942–952. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Ikeda, T. Oxidative Coupling of Methane over Alkali Chloride–Mn–Na2WO4/SiO2 Catalysts: Promoting Effect of Molten Alkali Chloride. Fuel Process. Technol. 2015, 133, 29–34. [Google Scholar] [CrossRef]
- Gordienko, Y.; Usmanov, T.; Bychkov, V.; Lomonosov, V.; Fattakhova, Z.; Tulenin, Y.; Shashkin, D.; Sinev, M. Oxygen Availability and Catalytic Performance of NaWMn/SiO2 Mixed Oxide and Its Components in Oxidative Coupling of Methane. Catal. Today 2016, 278, 127–134. [Google Scholar] [CrossRef]
- Matras, D.; Vamvakeros, A.; Jacques, S.D.M.; Grosjean, N.; Rollins, B.; Poulston, S.; Stenning, G.B.G.; Godini, H.R.; Drnec, J.; Cernik, R.J.; et al. Effect of Thermal Treatment on the Stability of Na-Mn-W/SiO2catalyst for the Oxidative Coupling of Methane. Faraday Discuss. 2021, 229, 176–196. [Google Scholar] [CrossRef] [Green Version]
- Pak, S.; Lunsford, J.H. Thermal Effects during the Oxidative Coupling of Methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO Catalysts. Appl. Catal. A Gen. 1998, 168, 131–137. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Matras, D.; Jacques, S.D.M.; di Michiel, M.; Middelkoop, V.; Cong, P.; Price, S.W.T.; Bull, C.L.; Senecal, P.; Beale, A.M. Real-Time Tomographic Diffraction Imaging of Catalytic Membrane Reactors for the Oxidative Coupling of Methane. Catal. Today 2021, 364, 242–255. [Google Scholar] [CrossRef]
- Yoon, S.; Lim, S.; Choi, J.W.; Suh, D.J.; Song, K.H.; Ha, J.M. Study on the Unsteady State Oxidative Coupling of Methane: Effects of Oxygen Species from O2, Surface Lattice Oxygen, and CO2 on the C2+ Selectivity. RSC Adv. 2020, 10, 35889–35897. [Google Scholar] [CrossRef]
- Tiemersma, T.P.; Tuinier, M.J.; Gallucci, F.; Kuipers, J.A.M.; Annaland, M.V.S. A Kinetics Study for the Oxidative Coupling of Methane on a Mn/Na2WO4/SiO2 Catalyst. Appl. Catal. A Gen. 2012, 433–434, 96–108. [Google Scholar] [CrossRef]
- Jones, C.A.; Leonard, J.J.; Sofranko, J.A. The Oxidative Conversion of Methane to Higher Hydrocarbons over Alkali-Promoted MnSiO2. J. Catal. 1987, 103, 311–319. [Google Scholar] [CrossRef]






| Name | Nomenclature | La Catalyst Mass Fraction in Bed |
|---|---|---|
| La/MgO | -- | |
| La2O3 | -- | |
| MnNaWSi | -- | |
| La2O3 + MnNaWSi | La2O3-MnNaWSi_0.1s | 0.1 |
| La/MgO + MnNaWSi | La/MgO-MnNaWSi_0.1s | 0.1 |
| La/MgO-MnNaWSi_0.2s | 0.2 | |
| La/MgO-MnNaWSi_0.5s | 0.5 | |
| La/MgO-MnNaWSi_0.1m | 0.1 | |
| La/MgO-MnNaWSi_0.2m | 0.2 | |
| La/MgO-MnNaWSi_0.8m | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Wang, J.; Shpasser, D.; Gazit, O.M. Probing Low-Temperature OCM Performance over a Dual-Domain Catalyst Bed. Chemistry 2023, 5, 1101-1112. https://doi.org/10.3390/chemistry5020075
Huang B, Wang J, Shpasser D, Gazit OM. Probing Low-Temperature OCM Performance over a Dual-Domain Catalyst Bed. Chemistry. 2023; 5(2):1101-1112. https://doi.org/10.3390/chemistry5020075
Chicago/Turabian StyleHuang, Baoting, Jin Wang, Dina Shpasser, and Oz M. Gazit. 2023. "Probing Low-Temperature OCM Performance over a Dual-Domain Catalyst Bed" Chemistry 5, no. 2: 1101-1112. https://doi.org/10.3390/chemistry5020075
APA StyleHuang, B., Wang, J., Shpasser, D., & Gazit, O. M. (2023). Probing Low-Temperature OCM Performance over a Dual-Domain Catalyst Bed. Chemistry, 5(2), 1101-1112. https://doi.org/10.3390/chemistry5020075