Thia-Michael Reaction under Heterogeneous Catalysis
Abstract
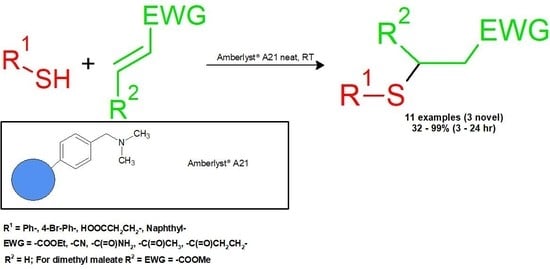
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Procedure
2.3. Product Identification
3. Results and Discussion
3.1. The Catalyst—Properties and Recyclability
3.2. E-Factor and Atom Economy of the Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Akanksha. Amino acid catalyzed thia-Michael addition reactions. Tetrahedron 2007, 63, 11086–11092. [Google Scholar] [CrossRef]
- Wadhwa, P.; Kharbanda, A.; Sharma, A. Thia-Michael addition: An emerging strategy in organic synthesis. Asian J. Org. Chem. 2018, 7, 634–661. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Lang, S.H.; Jiang, X. Sulphur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiol, I.V.; Allen, A.C.F.H.; Fournier, J.O.; Humphlett, W.J. The thermal reversibility of the Michael reaction. Can. J. Chem. 1964, 42, 2616–2620. [Google Scholar]
- Nicponski, D.; Marchi, J. Selectivity reversal during thia-Michael additions using tetrabutylammonium hydroxide: Operationally simple and extremely high turnover. Synthesis 2014, 46, 1725–1730. [Google Scholar] [CrossRef]
- Bandini, M.; Cozzi, P.G.; Giacomini, M.; Melchiorre, P.; Selva, S.; Umani-Ronchi, A. Sequential one-pot InBr3-catalyzed 1,4-then 1,2-nucleophilic addition to enones. J. Org. Chem. 2002, 67, 3700–3704. [Google Scholar] [CrossRef]
- Pore, D.M.; Soudagar, M.S.; Desai, U.V.; Thopate, T.S.; Wadagaonkar, P.P. Potassium phosphate or silica sulphuric acid catalyzed conjugate addition of thiols to α,β-unsaturated ketones at room temperature under solvent-free conditions. Tetrahedron Lett. 2006, 47, 9325–9328. [Google Scholar] [CrossRef]
- Wadhwa, P.; Bagchi, S.; Sharma, A. A Regioselective multicomponent cascade to access thiosemicarbazone-fused thiazinones: Scope, structure elucidation and gram scale synthesis. ChemistrySelect 2017, 2, 1386–1391. [Google Scholar] [CrossRef]
- Bosica, G.; Abdilla, R. Aza-Michael Mono-addition Using Acidic Alumina under Solventless Conditions. Molecules 2016, 21, 815. [Google Scholar] [CrossRef] [Green Version]
- Bosica, G.; Saliba, R. Aza-Michael Mono- and Bis-Addition of Primary and Secondary Amines Promoted by Silica-Supported Polyphosphoric Acid, PPA/SiO2. ChemistrySelect 2022, 7, e202104359. [Google Scholar] [CrossRef]
- Park, C.; Lee, S. One-pot sulfa-Michael addition reactions of disulfides using a pyridine-borane complex under blue light irradiation. Bull. Korean Chem. Soc. 2022, 43, 951–954. [Google Scholar] [CrossRef]
- Xu, X.B.; Yin, X.H.; Zhu, Y.Y.; Xu, X.H.; Luo, T.; Li, Y.H.; Lu, X.; Shao, L.L.; Pan, J.G.; Yang, R.H. Reduction cleavage of S-S bond by Zn/Cp2TiCl2: Application for the synthesis of β-arylthiocarbonyl compounds. J. Chem. Res. 2009, 12, 750–752. [Google Scholar] [CrossRef]
- Abbasi, M.; Nowrouzi, N.; Khezri, R. CuI-catalyzed tandem synthesis of thioethers using aryl halides, electron-deficient alkenes, and sodium iso-propyl xanthogenate. Appl. Organomet. Chem. 2020, 34, 1–9. [Google Scholar] [CrossRef]
- Hans, M.; Delaude, L.; Rodriguez, J.; Coquerel, Y. N-heterocyclic carbene catalyzed carba-, sulfa-, and phospha-Michael additions with NHC·CO2 adducts as precatalysts. J. Org. Chem. 2014, 79, 2758–2764. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Tarasenko, E.A.; Khokhlov, A.R.; Tyurin, V.S. Poly(N-vinylimidazole) as efficient and recyclable catalyst for the addition of thiols to Michael acceptors in aqueous medium. Russ. J. Org. Chem. 2007, 43, 1733–1736. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Bardajee, G.R.; Veranlou, R.O.C. KF/Al2O3-mediated Michael addition of thiols to electron-deficient olefins. Synth. Commun. 2005, 35, 2427–2433. [Google Scholar] [CrossRef]
- Payra, S.; Saha, A.; Banerjee, S. On-water magnetic NiFe2O 4 nanoparticle-catalyzed Michael additions of active methylene compounds, aromatic/aliphatic amines, alcohols and thiols to conjugated alkenes. RSC Adv. 2016, 6, 95951–95956. [Google Scholar] [CrossRef]
- Civit, M.G.; Sanz, X.; Vogels, C.M.; Webb, J.D.; Geier, S.J.; Decken, A.; Bo, C.; Westcott, S.A.; Fernández, E. Thioboration of α,β-unsaturated ketones and aldehydes toward the synthesis of β-sulfido carbonyl compounds. J. Org. Chem. 2015, 80, 2148–2154. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gorginpour, F.; Samadi, A. Dithiooxamide as an effective sulphur surrogate for odourless high-yielding carbon-sulphur bond formation in wet PEG200 as an eco-friendly, safe, and recoverable solvent. Eur. J. Org. Chem. 2015, 2015, 2914–2920. [Google Scholar] [CrossRef]
- Barbero, M.; Cadamuro, S.; Dughera, S. O-Benzenedisulfonimide as a reusable Brønsted acid catalyst for hetero-Michael reactions. Synth. Commun. 2013, 43, 758–767. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Krishnakumar, V.; Gunanathan, C. KOtBu-catalyzed Michael addition reactions under mild and solvent-free conditions. Chem. Asian J. 2020, 15, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Liu, Y.; Chai, L.; Lai, Z.; Fang, X.; Liu, B.; Zhang, W.; Lu, M.; Xu, Y.; Xu, H. Nmp-based ionic liquids: Recyclable catalysts for both hetero-Michael addition and Knoevenagel condensation in water. Synth. Commun. 2018, 48, 1060–1067. [Google Scholar] [CrossRef]
- Sigma, A. Amberlyst(R) A21 Free Base. Available online: https://www.sigmaaldrich.com/MT/en/product/aldrich/216410 (accessed on 6 February 2023).
- Bonfils, F.; Cazaux, I.; Hodge, P.; Caze, C. Michael reactions carried out using a bench-top flow system. Org. Biomol. Chem. 2006, 4, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Bosica, G.; Zammit, R. One-pot multicomponent nitro-Mannich reaction using a heterogeneous catalyst under solvent-free conditions. PeerJ 2018, 6, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.T.; Ghosh, S.; Choudhury, L.H. Perchloric acid impregnated on silica gel: A versatile catalyst for Michael addition of thiols to the electron-deficient alkenes. Eur. J. Org. Chem. 2006, 2006, 2226–2231. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Liu, L.; Xu, H.; Wang, Y. RuCl3 in poly(ethylene glycol): A highly efficient and recyclable catalyst for the conjugate addition of nitrogen and sulphur nucleophiles. Synthesis 2005, 13, 2129–2136. [Google Scholar] [CrossRef]







 | ||||
| Entry [a] | Catalyst [b] | T (°C) | Time (h) | Yield (%) of 3a [c] |
| 1 | Acid alumina | 50 | 2 | 94 |
| 2 | Neutral alumina | 50 | 2 | 95 |
| 3 | No | 50 | 2 | 47 |
| 4 | Basic alumina | 50 | 2 | 95 |
| 5 | Basic alumina | r.t | 3 | 90 |
| 6 | Amberlyst® A21 | r.t | 3 | 95 |
| 7 | Amberlyst® A15 | r.t | 3 | 40 |
| 8 | Montmorillonite K-10 | r.t | 3 | 55 |
| Catalyst Used | Solvent | Temperature/Time | Yield | Reusability | Reference |
|---|---|---|---|---|---|
| 0.5 mol% HClO4-SiO2 | CH2Cl2 | RT/10–15 min | 95% | 4 times (11% yield drop) | [26] |
| 0.5 mol% RuCl3 | PEG 2000 | 50 °C/8 h | 92% | 5 times (4% yield drop) | [27] |
| Amberlyst® A21 | Neat | RT/2 h | 95% | 5 times (12% yield drop) | - (this study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosica, G.; Abdilla, R.; Petrellini, A. Thia-Michael Reaction under Heterogeneous Catalysis. Organics 2023, 4, 86-96. https://doi.org/10.3390/org4010007
Bosica G, Abdilla R, Petrellini A. Thia-Michael Reaction under Heterogeneous Catalysis. Organics. 2023; 4(1):86-96. https://doi.org/10.3390/org4010007
Chicago/Turabian StyleBosica, Giovanna, Roderick Abdilla, and Alessio Petrellini. 2023. "Thia-Michael Reaction under Heterogeneous Catalysis" Organics 4, no. 1: 86-96. https://doi.org/10.3390/org4010007
APA StyleBosica, G., Abdilla, R., & Petrellini, A. (2023). Thia-Michael Reaction under Heterogeneous Catalysis. Organics, 4(1), 86-96. https://doi.org/10.3390/org4010007