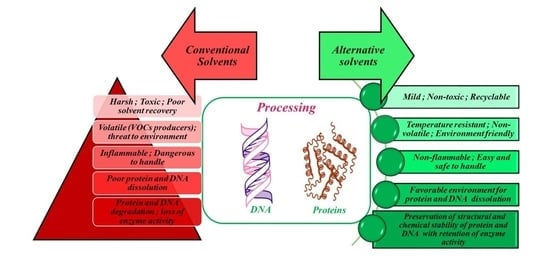
Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach
Abstract
:1. Introduction
2. Ionic Liquids for Biomacromolecules
2.1. Processing of Proteins in ILs
2.2. Ionic Liquids: From Solubility to Amplification of DNA
3. Deep Eutectic Solvents as Emerging Media for Green Technology
3.1. Deep Eutectic Solvents in Protein Processing
3.2. Deep Eutectic Solvents in DNA Processing
4. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [(C10)2NMDG-Br] | N,N-didecyl-N-methyl-d-glucaminium bromide |
| [2HEAA] | 2-hydroxyethyl ammonium acetate |
| [2-HEAF] | 2-Hydroxyethyl ammonium formate |
| [Ac] | acetate |
| [But] | butyl |
| [C16POHIM-Br] | 1-(1,2-dihydroxypropyl)-3-hexadecylimidazolium bromide |
| [C8mim] [C12OSO3] | 1-octyl-3-methyl imidazolium dodecyl sulfate |
| [C8mim] [Cl] | 1-octyl 3-methyl imidazolium chloride |
| [Ch] [A] | cholinium acetate |
| [Ch] [F] | cholinium formate |
| [Ch] | choline |
| [Ch] [DHP] | cholinium dihydrogen phosphate |
| [CHES] | 2-(cyclohexylamino)ethanesulfonate |
| [Cl] | chloride |
| [bmim] | 1-butyl 3-methyl imidazolium |
| [DEAA] | diethylammonium acetate |
| [DEAP] | diethyl ammonium dihydrogen phosphate |
| [DEAS] | diethyl ammonium hydrogen sulfate |
| [DH Cit] | dihydrogen citrate |
| [emim] | 1-ethyl 3-methyl imidazolium |
| [HEPES] | (4-(2-hydroxyethyl)-1-piperazineethanesulfonate) |
| [Lac] | lactate |
| [Mmim] [DMP] | N-methyl-N-methylimidazolium dimethyl phosphate |
| [Prop] | propyl |
| [Gly] | glycine |
| [TAcl] | tantalum chloride |
| [TEAA] | triethyl ammonium acetate |
| [TEAP] | triethyl ammonium dihydrogen phosphate |
| [TES] | 2-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]ethanesulfonate |
| [TMAA] | trimethyl ammonium acetate |
| [TMAP] | trimethyl ammonium dihydrogen phosphate |
| [TMAS] | trimethyl ammonium hydrogen sulfate |
| [Tricine] | N-(2-hydroxy-1,1-bis(hydroxymethyl)ethyl)glycinate |
| AE | atom economy |
| CE | carbon efficiency |
| ATPSs | aqueous two-phase systems |
| ATR | attenuated total reflectance |
| BMIM dca | 1-butyl 3-methyl imidazolium dicyanamide |
| BSA | bovine serum albumin |
| CD | circular dichroism |
| CHNS | carbon, hydrogen, nitrogen, sulfur |
| CT:α | chymotrypsin |
| Cytc | cytochrome C |
| DESs | deep eutectic solvents |
| DLS | dynamic light scattering |
| DNA | deoxyribonucleic acid |
| EMY | effective mass yield |
| E-factor | environmental factor |
| ESH | environment, safety, and health |
| FO | forward osmosis |
| FT-IR | Fourier-transform infrared |
| GCI-PR | Green Chemistry Institute Pharmaceutical Round Table |
| GFP | green fluorescence protein |
| GSK | Glaxo Smith Kline |
| HBAs | hydrogen bond acceptors |
| HBDs | hydrogen bond donors |
| Hy [Ch] [dhp] | hydrated cholinium dihydrogen phosphate |
| IgY | immunoglobulin Y |
| ILs | ionic liquids |
| ITC | isothermal calorimetry |
| iv | intrinsic viscosity |
| m.p. | melting point |
| NaDESs | natural deep eutectic solvents |
| NMR | nuclear magnetic resonance |
| PCR | polymerase chain reaction |
| PMI | process mass intensity |
| RME | reaction mass efficiency |
| PSD | post synaptic density |
| RID | radial immunodiffusion |
| SANS | small-angle neutron scattering |
| SDS PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SE-HPLC | size exclusion–high performance liquid chromatography |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| TPC | total plate count |
| Trp fluorescence | tryptophan fluorescence |
| U.V. | ultraviolet |
| UF | ultrafiltration |
| VOCs | volatile organic compounds |
| ε-PL | epsilon polylysine |
References
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, M. Green solvents for the dissolution and processing of biopolymers. Curr. Opin. Green Sustain. Chem. 2019, 18, 72–78. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Simone, P.; Lodge, T.P. Self-assembly of block copolymer micelles in an ionic liquid. J. Am. Chem. Soc. 2006, 128, 2745–2750. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Canongia Lopes, J.N.A.; Pádua, A.A.H. Nanostructural organization in ionic liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Ryu, D.D.Y.; Nam, D.-H. Biomolecular engineering: A new frontier in biotechnology. J. Mol. Catal. B Enzym. 2000, 10, 23–37. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Ballone, P. Room Temperature Ionic Liquids Meet Biomolecules: A Microscopic view of structure and dynamics. ACS Sustain. Chem. Eng. 2016, 4, 392–412. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, R.A.; Singh, N.; Pereira, M.M.; Chudasama, N.A.; Bhattacharya, S.; Sharma, M.; Mondal, D.; Prasad, K. High concentration solubility and stability of ɛ-poly-l-lysine in an ammonium-based ionic liquid: A suitable media for polypeptide packaging and biomaterial preparation. Int. J. Biol. Macromol. 2018, 120, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, R.A.; Sharma, M.; Pereira, M.M.; Singh, N.; Bhattacharya, S.; A Chudasama, N.; Prasad, K. One step selective partition of ε-polylysine present in broth cultures in ionic liquid-based aqueous biphasic systems. Sep. Sci. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Sequeira, R.A.; Dubey, S.; Pereira, M.M.; Maity, T.K.; Singh, S.; Mishra, S.; Prasad, K. Neoteric solvent systems as sustainable media for dissolution and film preparation of Poly-[(R)-3-hydroxybutyrate]. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Vidal, L.; Riekkola, M.-L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef]
- Soukup-Hein, R.J.; Warnke, M.M.; Armstrong, D.W. Ionic liquids in analytical chemistry. Annu. Rev. Anal. Chem. 2009, 2, 145–168. [Google Scholar] [CrossRef]
- Shahriari, S.; Tomé, L.C.; Araújo, J.M.M.; Rebelo, L.P.N.; Coutinho, J.A.P.; Marrucho, I.M.; Freire, M.G. Aqueous biphasic systems: A benign route using cholinium-based ionic liquids. RSC Adv. 2013, 3, 1835–1843. [Google Scholar] [CrossRef]
- Tang, B.; Bi, W.; Tian, M.; Row, K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B 2012, 904, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Row, K.; Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.J.H. Task-Specific Ionic Liquids. Chem. Lett. 2004, 33, 1072–1077. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Ionic liquid assisted enzymatic delignification of wood biomass: A new ‘green’ and efficient approach for isolating of cellulose fibers. Biochem. Eng. J. 2012, 60, 156–160. [Google Scholar] [CrossRef]
- Revie, F.; Muhammad, M.; Yoshimitsu, U. Enhanced enzymatic delignification of oil palm biomass with ionic liquid pretreatment. Biochem. Eng. J. 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Zhao, H. DNA stability in ionic liquids and deep eutectic solvents. J. Chem. Technol. Biotechnol. 2014, 90, 19–25. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamamoto, E.; Mannen, T.; Nagamune, T.; Nagamune, T. Protein refolding using chemical refolding additives. Biotechnol. J. 2013, 8, 17–31. [Google Scholar] [CrossRef]
- Patel, R.; Kumari, M.; Khan, A.B. Recent advances in the applications of ionic liquids in protein stability and activity: A review. Appl. Biochem. Biotechnol. 2014, 172, 3701–3720. [Google Scholar] [CrossRef]
- Taha, M.; Almeida, M.R.; Silva, F.A.; Domingues, P.; Ventura, S.P.; Coutinho, J.A.; Freire, M.G. Novel biocompatible and self-buffering ionic liquids for biopharmaceutical applications. Chem. Weinh. Bergstr. Ger. 2015, 21, 4781–4788. [Google Scholar] [CrossRef] [Green Version]
- Pretti, C.; Renzi, M.; Focardi, S.E.; Giovani, A.; Monni, G.; Melai, B.; Rajamani, S.; Chiappe, C. Acute toxicity and biodegradability of N-alkyl-N-methylmorpholinium and N-alkyl-DABCO based ionic liquids. Ecotoxicol. Env. Saf. 2011, 74, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of Ionic Liquids. CLEAN Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Morrissey, S.; Pegot, B.; Coleman, D.; Garcia, M.T.; Ferguson, D.; Quilty, B.; Gathergood, N. Biodegradable, non-bactericidal oxygen-functionalised imidazolium esters: A step towards ‘greener’ ionic liquids. Green Chem. 2009, 11, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitão, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids—Toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Stolte, S.; Matzke, M.; Arning, J.; Böschen, A.; Pitner, W.-R.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007, 9, 1170–1179. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [Green Version]
- Egorova, K.S.; Ananikov, V.P. Toxicity of ionic liquids: Eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360. [Google Scholar] [CrossRef]
- Wood, N.; Stephens, G. Accelerating the discovery of biocompatible ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1670–1674. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, H.; Zhao, B.; Liang, Z.; Zhang, L.; Zhang, Y. Advances of ionic liquids-based methods for protein analysis. TrAC Trends Anal. Chem. 2018, 108, 239–246. [Google Scholar] [CrossRef]
- Phillips, D.M.; Drummy, L.F.; Conrady, D.G.; Fox, U.M.; Naik, R.R.; Stone, M.O.; Trulove, P.C.; De Long, H.C.; Mantz, R.A. Dissolution and Regeneration ofBombyx moriSilk fibroin using ionic liquids. J. Am. Chem. Soc. 2004, 126, 14350–14351. [Google Scholar] [CrossRef]
- Wolski, P.W.; Clark, D.S.; Blanch, H.W. Green fluorescent protein as a screen for enzymatic activity in ionic liquid–aqueous systems for in situ hydrolysis of lignocellulose. Green Chem. 2011, 13, 3107–3110. [Google Scholar] [CrossRef]
- Pereira, M.M.; Pedro, S.N.; Quental, M.V.; Lima, A.S.; Coutinho, J.A.; Freire, M.G. Enhanced extraction of bovine serum albumin with aqueous biphasic systems of phosphonium- and ammonium-based ionic liquids. J. Biotechnol. 2015, 206, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Y.; Wang, J.; Wu, K.; Xuan, X.; Lu, X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep. Purif. Technol. 2009, 64, 288–295. [Google Scholar] [CrossRef]
- Kohno, Y.; Saita, S.; Murata, K.; Nakamura, N.; Ohno, H. Extraction of proteins with temperature sensitive and reversible phase change of ionic liquid/water mixture. Polym. Chem. 2011, 2, 862–867. [Google Scholar] [CrossRef]
- Biswas, A. Ionic liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydr. Polym. 2006, 66. [Google Scholar] [CrossRef]
- Choi, H.-M.; Kwon, I. Dissolution of Zein Using Protic Ionic Liquids:N-(2-Hydroxyethyl) Ammonium Formate andN-(2-Hydroxyethyl) Ammonium Acetate. Ind. Eng. Chem. Res. 2011, 50, 2452–2454. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [Green Version]
- Gibb, C.L.D.; Gibb, B.C. Anion Binding to Hydrophobic concavity is central to the salting-in effects of Hofmeister Chaotropes. J. Am. Chem. Soc. 2011, 133, 7344–7347. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H. Protein stabilization and enzyme activation in ionic liquids: Specific ion effects. J. Chem. Technol. Biotechnol. 2015, 91, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Venkatesu, P. Does the stability of proteins in ionic liquids obey the Hofmeister series? Int. J. Biol. Macromol. 2014, 63, 244–253. [Google Scholar] [CrossRef]
- Zhao, H. Methods for stabilizing and activating enzymes in ionic liquids—A review. J. Chem. Technol. Biotechnol. 2010, 85, 891–907. [Google Scholar] [CrossRef]
- van Rantwijk, F.; Sheldon, R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Yamaguchi, S.; Nagamune, T. Protein Refolding by N-Alkylpyridinium and N-Alkyl-N-methylpyrrolidinium Ionic Liquids. Appl. Biochem. Biotechnol. 2011, 164, 957–967. [Google Scholar] [CrossRef]
- Tariq, M.; Freire, M.G.; Saramago, B.; Coutinho, J.A.P.; Lopes, J.N.C.; Rebelo, L.P.N. Surface tension of ionic liquids and ionic liquid solutions. Chem. Soc. Rev. 2012, 41, 829–868. [Google Scholar] [CrossRef]
- Rogers, T.L.; Nelsen, A.C.; Sarkari, M.; Young, T.J.; Johnston, K.P.; Williams, R.O., 3rd. Enhanced aqueous dissolution of a poorly water soluble drug by novel particle engineering technology: Spray-freezing into liquid with atmospheric freeze-drying. Pharm. Res. 2003, 20, 485–493. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Kurnia, K.A.; Cojocaru, O.A.; Gurau, G.; Rebelo, L.P.N.; Rogers, R.D.; Freire, M.G.; Coutinho, J.A.P. Molecular interactions in aqueous biphasic systems composed of polyethylene glycol and crystalline vs. liquid cholinium-based salts. Phys. Chem. Chem. Phys. 2014, 16, 5723–5731. [Google Scholar] [CrossRef]
- Louros, C.L.S.; Cláudio, A.F.M.; Neves, C.M.S.S.; Freire, M.G.; Marrucho, I.M.; Pauly, J.; Coutinho, J.A.P. Extraction of biomolecules using phosphonium-based ionic liquids + K(3)PO(4) aqueous biphasic systems. Int. J. Mol. Sci. 2010, 11, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Pérez, M.; Tomé, L.I.N.; Freire, M.G.; Marrucho, I.M.; Cabeza, O.; Coutinho, J.A.P. (Extraction of biomolecules using) aqueous biphasic systems formed by ionic liquids and aminoacids. Sep. Purif. Technol. 2010, 72, 85–91. [Google Scholar] [CrossRef]
- Ito, Y.; Kohno, Y.; Nakamura, N.; Ohno, H. Design of phosphonium-type zwitterion as an additive to improve saturated water content of phase-separated ionic liquid from aqueous phase toward reversible extraction of proteins. Int. J. Mol. Sci. 2013, 14, 18350–18361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, S.P.M.; Silva, F.A.; Gonçalves, A.M.M.; Pereira, J.L.; Gonçalves, F.; Coutinho, J.A.P. Ecotoxicity analysis of cholinium-based ionic liquids to Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Santos, L.D.F.; Saraiva, J.A.; Coutinho, J.A.P. Ionic liquids microemulsions: The key to Candida antarctica lipase B superactivity. Green Chem. 2012, 14, 1620–1625. [Google Scholar] [CrossRef]
- Shimojo, K.; Nakashima, K.; Kamiya, N.; Goto, M. Crown ether-mediated extraction and functional conversion of cytochromecin ionic liquids. Biomacromolecules 2006, 7, 2–5. [Google Scholar] [CrossRef] [PubMed]
- 63. Shimojo, K.; Kamiya, N.; Tani, F.; Naganawa, H.; Naruta, Y.; Goto, M. Extractive solubilization, structural change, and functional conversion of cytochromecin ionic liquids via crown ether complexation. Anal. Chem. 2006, 78, 7735–7742. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kang, T.S. Ionic liquid surfactant mediated structural transitions and self-assembly of bovine serum albumin in aqueous media: Effect of functionalization of ionic liquid surfactants. J. Phys. Chem. B 2015, 119, 10573–10585. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ohno, H. Enzymatic activity and thermal stability of metallo proteins in hydrated ionic liquids. Biopolymers 2010, 93, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; MacFarlane, D.R.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and stability of cytochrome c in hydrated ionic liquids: Effect of Oxo acid residues and kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar] [CrossRef]
- Attri, P.; Venkatesu, P.; Kumar, A. Activity and stability of α-chymotrypsin in biocompatible ionic liquids: Enzyme refolding by triethyl ammonium acetate. Phys. Chem. Chem. Phys. 2011, 13, 2788–2796. [Google Scholar] [CrossRef]
- Quental, M.V.; Caban, M.; Pereira, M.M.; Stepnowski, P.; Coutinho, J.A.P.; Freire, M.G. Enhanced extraction of proteins using cholinium-based ionic liquids as phase-forming components of aqueous biphasic systems. Biotechnol. J. 2015, 10, 1457–1466. [Google Scholar] [CrossRef]
- Bharmoria, P.; Rao, K.S.; Trivedi, T.J.; Kumar, A. Biamphiphilic ionic liquid induced folding alterations in the structure of bovine serum albumin in aqueous medium. J. Phys. Chem. B 2014, 118, 115–124. [Google Scholar] [CrossRef]
- Heller, W.T.; O’Neill, H.M.; Zhang, Q.; Baker, G.A. Characterization of the influence of the ionic liquid 1-butyl-3-methylimidazolium chloride on the structure and thermal stability of green fluorescent protein. J. Phys. Chem. B 2010, 114, 13866–13871. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Xu, K.; Huang, Y.; Wen, Q.; Ding, X. Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Liu, M.; Chen, S.; Chen, X.; Wang, J. new insight into molecular interactions of imidazolium ionic liquids with bovine serum albumin. J. Phys. Chem. B 2011, 115, 12306–12314. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, A.; Edler, K.J.; Arnold, T.; Alba Venero, D.; Jackson, A.J. Protein conformation in pure and hydrated deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 8667–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, J.P.; Mc Cluskey, A.; Atkin, R. Activity and thermal stability of lysozyme in alkylammonium formate ionic liquids—Influence of cation modification. Green Chem. 2009, 11, 785–792. [Google Scholar] [CrossRef]
- Weaver, K.D.; Vrikkis, R.M.; Van Vorst, M.P.; Trullinger, J.; Vijayaraghavan, R.; Foureau, D.M.; McKillop, I.H.; MacFarlane, D.R.; Krueger, J.K.; Elliott, G.D. Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Phys. Chem. Chem. Phys. 2012, 14, 790–801. [Google Scholar] [CrossRef]
- Gelamo, E.L.; Tabak, M. Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2255–2271. [Google Scholar] [CrossRef]
- Sandoval, M.; Cortés, Á.; Civera, C.; Treviño, J.; Ferreras, E.; Vaultier, M.; Berenguer, J.; Lozano, P.; Hernáiz, M.J. Efficient and selective enzymatic synthesis of N-acetyl-lactosamine in ionic liquid: A rational explanation. RSC Adv. 2012, 2, 6306–6314. [Google Scholar] [CrossRef] [Green Version]
- Grudniewska, A.; de Melo, E.M.; Chan, A.; Gniłka, R.; Boratyński, F.; Matharu, A.S. enhanced protein extraction from oilseed cakes using glycerol–choline chloride deep eutectic solvents: A biorefinery approach. ACS Sustain. Chem. Eng. 2018, 6, 15791–15800. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Pelton, J.T.; McLean, L.R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef]
- Ismail, A.A.; Mantsch, H.H.; Wong, P.T.T. Aggregation of chymotrypsinogen: Portrait by infrared spectroscopy. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1992, 1121, 183–188. [Google Scholar] [CrossRef]
- Maiti, N.C.; Apetri, M.M.; Zagorski, M.G.; Carey, P.R.; Anderson, V.E. Raman spectroscopic characterization of secondary structure in natively unfolded proteins: Alpha-synuclein. J. Am. Chem. Soc. 2004, 126, 2399–2408. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Lewis, A.; Scheraga, H.A.; Saeva, F.D. Disulfide bond dihedral angles from Raman spectroscopy. Proc. Natl. Acad. Sci. USA 1973, 70, 2619–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, W.A.; Rubinstein, M.; Tan, J.S. Polyelectrolyte−Gelatin complexation: Light-scattering study. Macromolecules 1997, 30, 3262–3270. [Google Scholar] [CrossRef]
- Geng, F.; Zheng, L.; Liu, J.; Yu, L.; Tung, C. Interactions between a surface active imidazolium ionic liquid and BSA. Colloid Polym. Sci. 2009, 287, 1253–1259. [Google Scholar] [CrossRef]
- Nielsen, A.D.; Arleth, L.; Westh, P. Analysis of protein–surfactant interactions—A titration calorimetric and fluorescence spectroscopic investigation of interactions between Humicola insolens cutinase and an anionic surfactant. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1752, 124–132. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, S.; Wang, J.; Tang, J. Aggregation of ionic liquids [Cnmim] Br (n = 4, 6, 8, 10, 12) in D2O: A NMR Study. J. Phys. Chem. B 2008, 112, 2031–2039. [Google Scholar] [CrossRef]
- Cooper, A. Thermodynamic analysis of biomolecular interactions. Curr. Opin. Chem. Biol. 1999, 3, 557–563. [Google Scholar] [CrossRef]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef]
- Siamwiza, M.N.; Lord, R.C.; Chen, M.C.; Takamatsu, T.; Harada, I.; Matsuura, H.; Shimanouchi, T. Interpretation of the doublet at 850 and 830 cm−1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry 1975, 14, 4870–4876. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Joshi, M.D.; Ronning, D.R.; Anderson, J.L. Ionic liquids as solvents for in situ dispersive liquid-liquid microextraction of DNA. J. Chromatogr. A 2013, 1272, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jumbri, K.; Abdul Rahman, M.B.; Abdulmalek, E.; Ahmad, H.; Micaelo, N.M. An insight into structure and stability of DNA in ionic liquids from molecular dynamics simulation and experimental studies. Phys. Chem. Chem. Phys. 2014, 16, 14036–14046. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, R.; Izgorodin, A.; Ganesh, V.; Surianarayanan, M.; MacFarlane, D.R. Long-term structural and chemical stability of DNA in hydrated ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Sorensen, M.; Nacham, O.; Anderson, J.L. Preservation of DNA in nuclease-rich samples using magnetic ionic liquids. RSC Adv. 2016, 6, 39846–39851. [Google Scholar] [CrossRef]
- Chandran, A.; Ghoshdastidar, D.; Senapati, S. Groove binding mechanism of ionic liquids: A key factor in long-term stability of DNA in hydrated ionic liquids? J. Am. Chem. Soc. 2012, 134, 20330–20339. [Google Scholar] [CrossRef]
- Tateishi-Karimata, H.; Sugimoto, N. Structure, stability and behaviour of nucleic acids in ionic liquids. Nucleic Acids Res. 2014, 42, 8831–8844. [Google Scholar] [CrossRef]
- Clark, K.D.; Nacham, O.; Yu, H.; Li, T.; Yamsek, M.M.; Ronning, D.R.; Anderson, J.L. Extraction of DNA by magnetic ionic liquids: Tunable solvents for rapid and selective DNA analysis. Anal. Chem. 2015, 87, 1552–1559. [Google Scholar] [CrossRef]
- Clark, K.D.; Yamsek, M.M.; Nacham, O.; Anderson, J.L. Magnetic ionic liquids as PCR-compatible solvents for DNA extraction from biological samples. Chem. Commun. 2015, 51, 16771–16773. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, D.; Singh, N.; Trivedi, N.; Bhatt, J.; Prasad, K. High concentration DNA solubility in bio-ionic liquids with long-lasting chemical and structural stability at room temperature. RSC Adv. 2015, 5, 40546–40551. [Google Scholar] [CrossRef]
- Xuan, S.; Meng, Z.; Wu, X.; Wong, J.-R.; Devi, G.; Yeow, E.K.L.; Shao, F. Efficient DNA-mediated electron transport in ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 6703–6711. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, M.; Mondal, D.; Pereira, M.M.; Prasad, K. Very high concentration solubility and long-term stability of DNA in an ammonium-based ionic liquid: A suitable medium for nucleic acid packaging and preservation. ACS Sustain. Chem. Eng. 2017, 5, 1998–2005. [Google Scholar] [CrossRef]
- Pandey, P.K.; Rawat, K.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. Imidazolium based ionic liquid induced DNA gelation at remarkably low concentration. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 184–191. [Google Scholar] [CrossRef]
- Emaus, M.N.; Clark, K.D.; Hinners, P.; Anderson, J.L. Preconcentration of DNA using magnetic ionic liquids that are compatible with real-time PCR for rapid nucleic acid quantification. Anal. Bioanal. Chem. 2018, 410, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Clark, K.D.; Varona, M.; Emaus, M.N.; Anderson, J.L. Magnetic ionic liquid-enhanced isothermal nucleic acid amplification and its application to rapid visual DNA analysis. Anal. Chim. Acta 2019, 1045, 132–140. [Google Scholar] [CrossRef]
- Bowers, A.N.; Trujillo-Rodríguez, M.J.; Farooq, M.Q.; Anderson, J.L. Extraction of DNA with magnetic ionic liquids using in situ dispersive liquid–liquid microextraction. Anal. Bioanal. Chem. 2019, 411, 7375–7385. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.I.; Gonçalves, A.M.M.; Pereira, J.L.; Figueiredo, B.F.H.T.; Silva, F.A.; Coutinho, J.A.P.; Ventura, S.P.M.; Gonçalves, F. Environmental safety of cholinium-based ionic liquids: Assessing structure–ecotoxicity relationships. Green Chem. 2015, 17, 4657–4668. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Radošević, K.; Bubalo, M.C.; Srček, V.G.; Grgas, D.; Dragičević, T.L.; Redovniković, I.R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Liu, Q.P.; Smith, T.J.; Li, N.; Zong, M.H. Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids. PLoS ONE 2013, 8, e59145. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural deep eutectic solvent as extraction media for the main phenolic compounds from olive oil processing wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, W.; Mu, T. Controllable exfoliation of natural silk fibers into nanofibrils by protein denaturant deep eutectic solvent: Nanofibrous strategy for multifunctional membranes. Green Chem. 2018, 20, 3625–3633. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal. Chim. Acta 2015, 864, 9–20. [Google Scholar] [CrossRef]
- Mondal, D.; Bhatt, J.; Sharma, M.; Chatterjee, S.; Prasad, K. A facile approach to prepare a dual functionalized DNA based material in a bio-deep eutectic solvent. Chem. Commun. 2014, 50, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Mahto, A.; Veerababu, P.; Bhatt, J.; Prasad, K.; Nataraj, S.K. Deep eutectic solvents as a new class of draw agent to enrich low abundance DNA and proteins using forward osmosis. RSC Adv. 2015, 5, 89539–89544. [Google Scholar] [CrossRef]
- Mamajanov, I.; Engelhart, A.E.; Bean, H.D.; Hud, N.V. DNA and RNA in anhydrous media: Duplex, triplex, and G-quadruplex secondary structures in a deep eutectic solvent. Angew. Chem. Int. Ed. Engl. 2010, 49, 6310–6314. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.; Mondal, D.; Bhojani, G.; Chatterjee, S.; Prasad, K. Preparation of bio-deep eutectic solvent triggered cephalopod shaped silver chloride-DNA hybrid material having antibacterial and bactericidal activity. Mater. Sci. Eng. C 2015, 56, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, C.; Prasad, K. Formation of multiple structural formats of DNA in a Bio-Deep eutectic solvent. Macromol. Chem. Phys. 2015, 216, 1061–1066. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Dai, Q.; Zhou, Y. Magnetic deep eutectic solvents molecularly imprinted polymers for the selective recognition and separation of protein. Anal. Chim. Acta 2016, 936, 168–178. [Google Scholar] [CrossRef]
- 127. Lee, M.S.; Lee, K.; Nam, M.W.; Jeong, K.M.; Lee, J.E.; Kim, N.W.; Yin, Y.; Lim, S.Y.; Yoo, D.E.; Lee, J.; et al. Natural deep eutectic solvents as a storage medium for human interferon-α2: A green and improved strategy for room-temperature biologics. J. Ind. Eng. Chem. 2018, 65, 343–348. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Vilela, C.; Pinto, R.J.B.; Martins, M.A.; Marrucho, I.M.; Freire, C.S.R. Tuning lysozyme nanofibers dimensions using deep eutectic solvents for improved reinforcement ability. Int. J. Biol. Macromol. 2018, 115, 518–527. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P.; Schrebler, R.A. Remarkable stability of Candida antarctica lipase B immobilized via cross-linking aggregates (CLEA) in deep eutectic solvents. Biocatal. Biotransformation 2019, 37, 106–114. [Google Scholar] [CrossRef]
- Zhao, C.; Ren, J.; Qu, X. G-Quadruplexes form ultrastable parallel structures in deep eutectic solvent. Langmuir 2013, 29, 1183–1191. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Mukesh, C.; Gupta, V.; Prasad, K. Improved solubility of DNA in recyclable and reusable bio-based deep eutectic solvents with long-term structural and chemical stability. Chem. Commun. 2013, 49, 9606–9608. [Google Scholar] [CrossRef] [PubMed]
- Gállego, I.; Grover, M.A.; Hud, N.V. Folding and imaging of DNA nanostructures in anhydrous and hydrated deep-eutectic solvents. Angew. Chem. Int. Ed. 2015, 54, 6765–6769. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Y.; Xu, K.; Wen, Q.; Ding, X.; Zhang, H.; Yang, Q. High-performance of deep eutectic solvent based aqueous bi-phasic systems for the extraction of DNA. RSC Adv. 2016, 6, 84406–84414. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Chen, J.; Wei, X.; Xu, W.; Ni, R.; Meng, J.; Zhou, Y. A novel aqueous biphasic system formed by deep eutectic solvent and ionic liquid for DNA partitioning. Talanta 2018, 189, 467–479. [Google Scholar] [CrossRef]






| Protein | Source | Type | ILs | Bioprocessing Outcome | Techniques for Bioprocessing | Ref. |
|---|---|---|---|---|---|---|
| IgY | Egg yolk | Antibody | [Ch]X X = [ME] [HEPES, [Tricine] [TES] or [CHES] | Extraction anandd purification of IgY from egg yolk using GB-ILs based ATPSs | TPC, RID, SDS-PAGE, PSD, UF | [30] |
| Zein | Maize | Prolamins | BMIMC, BMIMdca | 10 wt% of zein soluble at 80 °C | FT-IR, ATR, NMR, iv | [45] |
| Cyt c, peroxidase, azurin, pseudoazurin, ascorbate oxidase, fructose dehydrogenase | Cells of animals and plants | Metalloproteins | Hy[ch] [dhp] | All metalloproteins were soluble in IL above 1 mM concentration with thermal stability without losing their enzyme activity. | UV, CD, RRS | [65] |
| Cytochrome C | Horse heart | Heme protein | [Ch] [dh] | Thermal and structural stability maintained up to 18 months in IL | ATR-FTIR, UV, RRS | [66] |
| CT | Bovine pancreas Type II | Pancreatic enzyme | [TEAA], [TEAP], [TMAS] [TMAA], [DEAA] [DEAS], [DEAP] | Stability of tertiary structure. For TMAS 80 °C and TMAP 85 °C | NMR, CD, UV, Near-UV CD | [67] |
| BSA | Derived from Cow | Blood Albumin | [Ch] [X] and X = [Lac], [TACl], [DHCit], [Ac], [Bit], [DHP], [Prop], [Gly] or [But] | Single step complete extraction (92–100%) of BSA using ILs maintaining its activity and stability. | ATPSs | [68] |
| BSA | Derived from Cow | Blood Albumin | [C8mim] [C12OSO3] | IL unfolds BSA under its CMC and refolds above its CAC | CD, Fluorimetry, DLS, ITC | [69] |
| BSA | Derived from Cow | Blood Albumin | [Ch] [X] and X = [Cl], [TES], [Tricine] or [HEPES] | α-helical structure maintained in all ILs | ATPSs, SE-HPLC, UV, CD, RP-HPLC, DLS, ATR-FTIR, Molecular Docking | [30] |
| GFP | A. Victoria jellyfish | Fluorescent protein | [bmim] [Cl] | Unfolding of protein from its native structure and decrease of thermal stability in the presence of IL | CD, fluorescence spectroscopy | [70] |
| GFP | B. Victoria jelly fish | Fluorescent protein | [Emim] [Lac], [Mmim] [DMP] | ILs proved to be potential candidates for the in situ enzymatic hydrolysis | Fluorescence spectroscopy | [41] |
| ε-PL | Streptomyces albulus | Polypeptide | [2HEA]], [2HEA] [A] [Ch] [F] and [Ch] [A] | The polypeptide was soluble at RT in all ILs and showed gelation in [2-HEA] [F]. | NMR, CD, IR, UV | [15,16] |
| Techniques | Characterization | Evaluation | Ref. |
|---|---|---|---|
| UV–vis | The native state of protein and complexation of it with ILs/DESs | Perturbation of polypeptide backbone and immobilization degree of aromatic amino acid residues | [67,71,72] |
| Near-UV CD | Protein tertiary structure | Degree of aromatic amino acid residues | [71,73,74,75] |
| Far-UV CD | Secondary structure of protein | Protein backbone perturbation | [73,76,77] |
| NMR | Protein Conformation in the presence of ILs/DESs | Changes of chemical shifts in ILs/DESs presence | [67,78] |
| FT-IR | Protein secondary structure | Analysis of peak position of Amide I and hydrogen bonding pattern | [29,71,78,79,80,81] |
| Raman spectroscopy | Protein secondary structure | Analysis of peak position of Amide I and III due to the binding of the protein with ILs/DESs | [82,83] |
| Raman spectroscopy | The tertiary structure of the protein | Skeletal bending, C–C–N, S–S and the C–S stretching frequencies of the disulphide bonds. | [74,75] |
| DLS | Size and structure of the protein in the presence of ILs/DESs | Hydrodynamic radii of protein changes as a result of protein–ILs/DESs interaction | [71,84,85] |
| Microcalorimetry | Binding stoichiometry | The exothermic enthalpy change for electrostatic interaction and endothermic enthalpy change for unfolding. | [53,86] |
| SANS | Shape, Size and structure of protein in the presence of ILs/DESs | Deuterate ILs/DESs or proteins required for information of either component. Neutron source required e.g., Swiss Palliation Source | [73,87,88] |
| Trp fluorescence | Protein tertiary structure and complexation of it with ILs/DESs | Variation of Trp microenvironment due to ILs/DESs binding with solvent and change in protein conformation | [69,89,90] |
| CHNS | Elemental composition of protein in ILs/DESs presence | Variation of the elemental composition of protein due to ILs/DESs bioprocessing | [78] |
| SDS PAGE | Separation of polypeptide bands based on their molar mass | Polypeptide bands perturbation | [78] |
| TGA | Protein degradation temperature | Changes in protein degradation rate due to ILs/DESs | [78] |
| Techniques | Characterization | Evaluation | Ref. |
|---|---|---|---|
| CD | The helical structure of DNA in presence of ILS/DESs | Perturbation of the helical structure of DNA in ILs/DESs presence | [131] |
| UV–vis | The native state of DNA and complexation of its minor and major grooves with ILs/DESs | Perturbation in absorbance based on the amount of complexation with ILs/DESs | [96] |
| ITC | Binding parameters of major and minor grooves of DNA with ILs/DESs | Changes in binding in presence of ILs/DESs | [103] |
| PCR Amplification | Order of DNase sequence | The sequence during amplification changes in presence of ILs/DESs shows degradation | [48] |
| Agarose gel electrophoresis | Visualization of intact bands of DNA | Degradation of DNA in presence of ILs/DESs leads to disintegrated bands | [131] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeira, R.A.; Bhatt, J.; Prasad, K. Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach. Sustain. Chem. 2020, 1, 116-137. https://doi.org/10.3390/suschem1020010
Sequeira RA, Bhatt J, Prasad K. Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach. Sustainable Chemistry. 2020; 1(2):116-137. https://doi.org/10.3390/suschem1020010
Chicago/Turabian StyleSequeira, Rosy Alphons, Jitkumar Bhatt, and Kamalesh Prasad. 2020. "Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach" Sustainable Chemistry 1, no. 2: 116-137. https://doi.org/10.3390/suschem1020010
APA StyleSequeira, R. A., Bhatt, J., & Prasad, K. (2020). Recent Trends in Processing of Proteins and DNA in Alternative Solvents: A Sustainable Approach. Sustainable Chemistry, 1(2), 116-137. https://doi.org/10.3390/suschem1020010