Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Plasma Treatment
2.3. Atmospheric Pressure Plasma Treatment
2.4. Analytical Methods
3. Results and Discussion
3.1. Decomposition of Alcohols in Solution Plasma
3.2. Decomposition of Alcohols in Atmospheric Pressure Plasma
3.3. Plasma Emission Spectroscopy
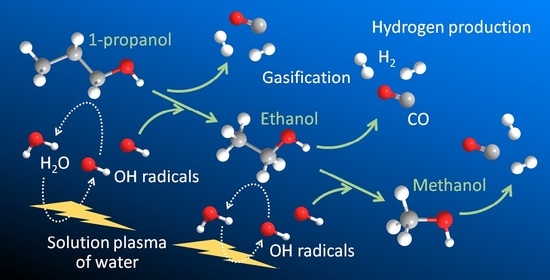
3.4. Proposed Decomposition Pathway of Alcohols in Solution Plasma
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B

Appendix C


References
- Palanisamy, A.; Soundarrajan, N.; Ramasamy, G. Analysis on production of bioethanol for hydrogen generation. Environ. Sci. Pollut. Res. 2021, 28, 63690–63705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]
- Inayat, A.; Tariq, R.; Khan, Z.; Ghenai, C.; Kamil, M.; Jamil, F.; Shanableh, A. A comprehensive review on advanced thermochemical processes for bio-hydrogen production via microwave and plasma technologies. Biomass Convers. Biorefinery 2020, 1–10. [Google Scholar] [CrossRef]
- Arpia, A.A.; Nguyen, T.B.; Chen, W.H.; Dong, C.D.; Ok, Y.S. Microwave-assisted gasification of biomass for sustainable and energy-efficient biohydrogen and biosyngas production: A state-of-the-art review. Chemosphere 2022, 287, 132014. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, L.; Mlotek, M.; Krawczyk, K. Efficient Plasma Technology for the Production of Green Hydrogen from Ethanol and Water. Energies 2022, 15, 2777. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Zike, Q.; Xiange, W.; JianMin, M.; JiaMin, D.; ChangMing, D. Green hydrogen from bio-ethanol reforming using micro plasma. Waste Dispos. Sustain. Energy 2020, 2, 15. [Google Scholar]
- Du, C.M.; Li, H.X.; Zhang, L.; Wang, J.; Huang, D.W.; Xiao, M.D.; Cai, J.W.; Chen, Y.B.; Yan, H.L.; Xiong, Y. Hydrogen production by steam-oxidative reforming of bio-ethanol assisted by Laval nozzle arc discharge. Int. J. Hydrogen Energy 2012, 37, 8318–8329. [Google Scholar] [CrossRef]
- Du, C.M.; Mo, J.M.; Li, H.X. Renewable Hydrogen Production by Alcohols Reforming Using Plasma and Plasma-Catalytic Technologies: Challenges and Opportunities. Chem. Rev. 2015, 115, 1503–1542. [Google Scholar] [CrossRef]
- Takai, O. Solution plasma processing (SPP). Pure Appl. Chem. 2008, 80, 2003–2011. [Google Scholar] [CrossRef]
- Akyuz, A.; Ozkan, M. Degradation of Polyvinylpyrrolidone by Solution Plasma Process. Acta Phys. Pol. A 2017, 131, 343–345. [Google Scholar] [CrossRef]
- Mun, M.K.; Lee, W.O.; Park, J.W.; Kim, D.S.; Yeom, G.Y.; Kim, D.W. Nanoparticles Synthesis and Modification using Solution Plasma Process. Appl. Sci. Converg. Technol. 2017, 26, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Hieda, J.; Shirafuji, T.; Noguchi, Y.; Saito, N.; Takai, O. Solution Plasma Surface Modification for Nanocarbon-Composite Materials. J. Jpn. Inst. Met. 2009, 73, 938–942. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Sato, M.; Clements, J.S. Optical study of active species produced by a pulsed streamer corona discharge in water. J. Electrost. 1997, 39, 189–202. [Google Scholar] [CrossRef]
- Xin, Y.B.; Sun, B.; Zhu, X.M.; Yan, Z.Y.; Liu, Y.J.; Liu, H. Characteristics of hydrogen produced by pulsed discharge in ethanol solution. Appl. Energy 2016, 168, 122–129. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.T.; Xin, Y.B.; Zhu, X.M. Large capacity hydrogen production by microwave discharge plasma in liquid fuels ethanol. Int. J. Hydrogen Energy 2017, 42, 24047–24054. [Google Scholar] [CrossRef]
- Zhu, T.H.; Sun, B.; Zhu, X.M.; Wang, L.R.; Xin, Y.B.; Liu, J.L. Mechanism analysis of hydrogen production by microwave discharge in ethanol liquid. J. Anal. Appl. Pyrolysis 2021, 156, 105111. [Google Scholar] [CrossRef]
- Wang, B.; Sun, B.; Zhu, X.M.; Yan, Z.Y.; Liu, Y.J.; Liu, H.; Liu, Q. Hydrogen production from alcohol solution by microwave discharge in liquid. Int. J. Hydrogen Energy 2016, 41, 7280–7291. [Google Scholar] [CrossRef]
- Xin, Y.B.; Sun, B.; Zhu, X.M.; Yan, Z.Y.; Zhao, X.T.; Sun, X.H. Hydrogen production from ethanol decomposition by pulsed discharge with needle-net configurations. Appl. Energy 2017, 206, 126–133. [Google Scholar] [CrossRef]
- Zhao, X.T.; Sun, B.; Zhu, T.H.; Zhu, X.M.; Yan, Z.Y.; Xin, Y.B.; Sun, X.H. Pathways of hydrogen-rich gas produced by microwave discharge in ethanol-water mixtures. Renew. Energy 2020, 156, 768–776. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhou, R.S.; Qi, F.; Zhou, D.J.; Zhou, R.W.; Wan, J.J.; Xian, Y.B.; Cullen, P.J.; Lu, X.P.; Ostrikov, K. Plasma-enabled liquid ethanol conversion for hydrogen production: Discharge characteristics and process control. J. Phys. D-Appl. Phys. 2020, 53, 174001. [Google Scholar] [CrossRef]
- Franclemont, J.T.; Fan, X.R.; Li, R.; Singh, R.K.; Holsen, T.M.; Thagard, S.M. Chemical reaction mechanisms accompanying pulsed electrical discharges in liquid methanol. Plasma Process. Polym. 2018, 15, 1800019. [Google Scholar] [CrossRef]
- Shiraishi, R.; Nomura, S.; Mukasa, S.; Nakano, R.; Kamatoko, R. Effect of catalytic electrode and plate for methanol decomposition by in-liquid plasma. Int. J. Hydrogen Energy 2018, 43, 4305–4310. [Google Scholar] [CrossRef]
- Xin, Y.B.; Sun, B.; Zhu, X.M.; Yan, Z.Y.; Liu, H.; Liu, Y.J. Effects of plate electrode materials on hydrogen production by pulsed discharge in ethanol solution. Appl. Energy 2016, 181, 75–82. [Google Scholar] [CrossRef]
- Xin, Y.B.; Wang, Q.L.; Sun, J.B.; Sun, B. Plasma in aqueous methanol: Influence of plasma initiation mechanism on hydrogen production. Appl. Energy 2022, 325, 119892. [Google Scholar] [CrossRef]
- Minami, E.; Miyamoto, T.; Kawamoto, H. Decomposition of Saccharides and Alcohols in Solution Plasma for Hydrogen Production. Hydrogen 2022, 3, 339–347. [Google Scholar] [CrossRef]
- Susanti, R.F.; Dianningrum, L.W.; Yum, T.; Kim, Y.; Lee, Y.W.; Kim, J. High-yield hydrogen production by supercritical water gasification of various feedstocks: Alcohols, glucose, glycerol and long-chain alkanes. Chem. Eng. Res. Des. 2014, 92, 1834–1844. [Google Scholar] [CrossRef]
- Daidoji, H. Kaen wo mochiita bunkou-bunseki gijutsu (Flame Spectroscopic Analysis Technique). In Kaen no Bunkougakuteki-Keisoku to Sono Ouyo (Spectroscopic Measurement of Flames and Its Applications), 1st ed.; Koda, S., Takubo, Y., Eds.; Japan Scientific Societies Press: Tokyo, Japan, 1990; pp. 111–159. (In Japanese) [Google Scholar]









| Alcohol Concentration (wt%) | H2/CO Ratio (mol/mol) | |||
|---|---|---|---|---|
| MeOH | EtOH | 1-PrOH | 2-PrOH | |
| 3 | 2.24 | 2.02 | 1.74 | 1.60 |
| 10 | 1.79 | 1.74 | 1.76 | 1.52 |
| 21 | 1.69 | 1.86 | 1.90 | 1.43 |
| 32 | 1.99 | 1.67 | 1.79 | 1.41 |
| 44 | 1.62 | 1.89 | 2.02 | 1.59 |
| 57 | 1.59 | 1.61 | 1.84 | 1.57 |
| 70 | 1.55 | 1.90 | 1.57 | 1.62 |
| Alcohol Concentration (wt%) | O/C Ratio (mol/mol) of the Product Gas | |||
|---|---|---|---|---|
| MeOH (O/C = 1.00) | EtOH (O/C = 0.50) | 1-PrOH (O/C = 0.33) | 2-PrOH (O/C = 0.33) | |
| 3 | 1.09 | 0.95 | 0.87 | 0.99 |
| 10 | 1.07 | 0.93 | 0.90 | 0.86 |
| 21 | 1.02 | 0.88 | 0.85 | 0.82 |
| 32 | 1.01 | 0.83 | 0.81 | 0.77 |
| 44 | 0.96 | 0.82 | 0.75 | 0.73 |
| 57 | 0.97 | 0.78 | 0.74 | 0.73 |
| 70 | 0.96 | 0.74 | 0.67 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, T.; Minami, E.; Kawamoto, H. Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism. Hydrogen 2023, 4, 373-388. https://doi.org/10.3390/hydrogen4020026
Miyamoto T, Minami E, Kawamoto H. Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism. Hydrogen. 2023; 4(2):373-388. https://doi.org/10.3390/hydrogen4020026
Chicago/Turabian StyleMiyamoto, Takaki, Eiji Minami, and Haruo Kawamoto. 2023. "Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism" Hydrogen 4, no. 2: 373-388. https://doi.org/10.3390/hydrogen4020026
APA StyleMiyamoto, T., Minami, E., & Kawamoto, H. (2023). Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism. Hydrogen, 4(2), 373-388. https://doi.org/10.3390/hydrogen4020026