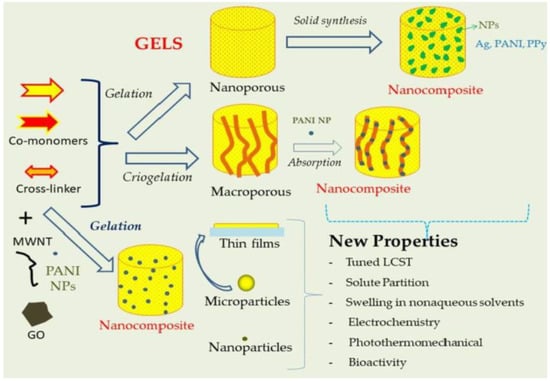
Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications
Abstract
:1. Introduction
- (i)
- Gels can be formed by homopolymerization or copolymerization of any acrylamide, and the relative reactivity of the comonomers is always the same, whatever is the functional group attached to the amide.
- (ii)
- Any acrylamide can be polymerized (together with a cross-linker) into a solid hydrogel using the same initiator (e.g., APS/TEMED).
- (iii)
- The incorporation of a linear conductive polymer inside the gel by in situ polymerization produces a semi-interpenetrated network.
- (iv)
- The swelling of the hydrogel intake water and ions dissolved in it, in the same concentration as in solution. Conversely, if the gel collapses (e.g., by reaching LCST), water is expelled and the ions are loaded inside.
- (v)
- If the gel contains fixed charged groups, the mobile counterions are taken into the network and the co-ions are excluded (Donnan exclusion).
- (vi)
- Macroporous hydrogels are mechanically weaker than their nanoporous counterparts due to the lower percentage of solid material.
- (vii)
- Gels, as solids, have defined interfaces with the solution.
- (viii)
- Hydrogels swell in water and similar solvents (e.g., alcohols) but not in low-polarity solvents (e.g., chloroform).
- (ix)
- Loading of substances to be released has to be performed from aqueous solution, even for molecules almost insoluble in water.
- (x)
- It is not possible to trap biological entities (enzymes, cells) by in situ radical polymerization/gelation since radicals themselves and/or acrylamides themselves are deleterious to those entities.
2. Summary of Experimental Procedures
2.1. Macroscopic Gels
2.1.1. Nanoporous
2.1.2. Macroporous
Directional Cryogelation
Gas Templating
2.2. Thin Films
2.3. Nanoparticles
2.3.1. Nanogels
2.3.2. Smart Nanoparticles Stabilized by Polyacrylamides
2.3.3. Block Copolymers of Acrylamides and Anilines
2.4. Microparticles
2.5. Nanocomposites
2.5.1. With Metallic Nanoparticles
2.5.2. With Conductive Polymer Nanoparticles
Filling the Pores by In Situ Polymerization/Precipitation
Trapping of Preformed Nanoparticles
2.5.3. Graphene
2.5.4. Carbon Nanotubes
2.5.5. Enzymes
2.5.6. Yeast Cells
2.6. Semi-Interpenetrated Networks (s-IPN)
2.7. Microscopy
2.8. Absorption of Solvents, Equilibrium, and Kinetics
2.9. Porosity
2.10. Lower Critical Solution Temperature (LCST)
2.11. Mechanical Properties
2.12. Contact Angle
2.13. Biocompatibility/Toxicity
3. Results and Discussion
3.1. Fabrication of Different Shapes/Forms
3.1.1. Nanoporous Gels
3.1.2. Macroporous Gels
3.1.3. Thin Films and Submicrometric Structures
3.1.4. Nanogels and Microgels
3.1.5. Gels as Nanocomposite Matrixes
3.1.6. True Semi-Interpenetrated Networks (s-IPN)
3.2. Tuning the Physicochemical Properties of the Gels
3.2.1. Swelling in Water
3.2.2. Swelling in Nonaqueous Solvents (and Mixtures)
3.2.3. Lower Critical Solution Temperatures (LCST)
3.2.4. Solute Partition
3.2.5. Mass Transport of Mobile Species
Mechanical Properties
3.2.6. Hydrophilicity
3.2.7. Photothermomechanical Behavior
3.3. Applications
3.3.1. Drug Release
3.3.2. Actuators
3.3.3. Sensors
3.3.4. Biological Systems
3D Cell Scaffolds
Immobilization of Bioactive Agents
Cell Adhesion Surfaces
Carriers for Active Principles
Antimicrobial Gels
4. Conclusions
5. Future Studies and Endeavors
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary of Abbreviations
| 2-MeTHF | 2-methyltetrahydrofuran |
| 4-ATF | 4-aminothiophenol |
| AA | acrylic acid |
| AAm | acrylamide |
| ACN | acetonitrile |
| AMPS | 2-acrylamido-2-methylpropanesulfonic acid |
| AMY | α-amylase |
| AMY@PAAm-GO | α-amylose in PAAm-GO |
| ANI | aniline |
| APTMAC | (3-acrylamidopropyl)trimethylammonium chloride |
| BPhen | bathophenanthroline |
| CP | conducting polymer |
| DAMAC | diallyldimethylammonium chloride |
| DLIP | direct laser interference patterning |
| DLS | dynamic light scattering |
| DMF | dimethylformamide |
| GO | graphene oxide |
| HBT | human body temperature |
| HEA | N-hydroxyethylacrylamide |
| HMA | N-acryloyl-tris-(hydroxymethyl)aminomethane |
| HPC | hydroxypropylcellulose |
| IgY | egg yolk immunoglobulin |
| LCST | lower critical solution temperature |
| MMA | methylmethacrylate |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MWNTs | multiwall carbon nanotubes |
| NAT | N-[Tris(hydroxymethyl)methyl]acrylamide |
| NIPAM | N-isopropylacrylamide |
| NIR | near infrared |
| NMP | N-methylpyrrolidone |
| PAAm | polyacrylamide |
| PANI | polyaniline |
| Pc | partition coefficient |
| PC | propylene carbonate |
| PEDOT | poly(ethylenedioxythiophene) |
| Phen | phenanthroline |
| PMMA | poly(methylmethacrylate) |
| PNMANI | poly(N-methylaniline) |
| PNNDMA | poly(N,N-dimethylacrylamide) |
| PPy | polypyrrole |
| rGO | reduced graphene oxide |
| Rubipy | Ru(bypyridine)32+ |
| SDS | sodium dodecyl sulfate |
| SEM | scanning electron microscopy |
| s-IPN | semi-interpenetrating network |
| SPAN 80 | sorbitan monooleate |
| TAA+ | tetraalkylammonium ion |
| TEMED | N,N,N′,N′-tetramethylethylenediamine |
| TPB− | tetraphenylborate |
| VOC | volatile organic contaminant |
Appendix A
| Mixture | xwater | xEtOH | xtoluene |
|---|---|---|---|
| M1 | 0.6341 | 0.3539 | 0.01 |
| M2 | 0.446 | 0.504 | 0.05 |
| M3 | 0.3375 | 0.5625 | 0.1 |
| M4 | 0.2603 | 0.5397 | 0.2 |
| M5 | 0.1048 | 0.3952 | 0.5 |
| M7 | 0.0404 | 0.2696 | 0.7 |
References
- Yang, T.-H. Recent Applications of Polyacrylamide as Biomaterials. Recent Pat. Mater. Sci. 2008, 1, 29–40. [Google Scholar] [CrossRef]
- Osada, Y.; Khokhlov, A. Polymer Gels and Networks; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Available online: https://polymer.bocsci.com/products/acrylamide (accessed on 7 March 2022).
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.J.; Doroudiani, S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Darnell, M.C.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and Biocompatibility of Extremely Tough Alginate/Polyacrylamide Hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Baumann, G.; Chrambach, A. A highly crosslinked, transparent polyacrylamide gel with improved mechanical stability for use in isoelectric focusing and isotachophoresis. Anal. Biochem. 1976, 70, 32–38. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.-F.; Zhao, C.-S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155. [Google Scholar] [CrossRef]
- Rivarola, C.R.; Biasutti, M.A.; Barbero, C.A. A visible light photoinitiator system to produce acrylamide based smart hydrogels: Ru(bpy)3+2 as photopolymerization initiator and molecular probe of hydrogel microenvironments. Polymer 2009, 50, 3145–3152. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Evidence of hydrophobic interactions controlling mobile ions release from smart hydrogels. Mol. Cryst. Liq. Cryst. 2010, 521, 265–271. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Miras, M.C.; Lescano, D.; Barbero, C.A. Nanocomposite synthesis by absorption of nanoparticles into macroporous hydrogels. Building a chemomechanical actuator driven by electromagnetic radiation. Nanotechnology 2011, 22, 245504. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Effect of copolymerization and semi-interpenetration with conducting polyanilines on the physicochemical properties of poly(N-isopropylacrylamide) based thermosensitive hydrogels. Eur. Polym. J. 2011, 47, 1977–1984. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Broglia, M.F.; Acevedo, D.F.; Barbero, C.A. Smart surfaces: Reversible switching of a polymeric hydrogel topography. Soft Matter 2012, 8, 307–310. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Study on partition and release of molecules in superabsorbent thermosensitive nanocomposites. Polymer 2012, 53, 445–453. [Google Scholar] [CrossRef]
- Rivero, R.E.; Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Pressure and microwave sensors/actuators based on smart hydrogel/conductive polymer nanocomposite. Sens. Actuators B Chem. 2014, 190, 270–278. [Google Scholar] [CrossRef]
- Bellingeri, R.; Alustiza, F.; Picco, N.; Acevedo, D.; Molina, M.A.; Rivero, R.; Grosso, C.; Motta, C.; Barbero, C.; Vivas, A. In vitro toxicity evaluation of hydrogel-carbon nanotubes composites on intestinal cells. J. Appl. Polym. Sci. 2015, 132, 41370. [Google Scholar] [CrossRef]
- Bellingeri, R.V.; Picco, N.Y.; Alustiza, F.E.; Canova, J.V.; Molina, M.A.; Acevedo, D.F.; Barbero, C.; Vivas, A.B. pH-responsive hydrogels to protect IgY from gastric conditions: In vitro evaluation. J. Food Sci. Technol. 2015, 52, 3117–3122. [Google Scholar] [CrossRef]
- Martínez, M.V.; Abel, S.B.; Rivero, R.; Miras, M.C.; Rivarola, C.R.; Barbero, C.A. Polymeric nanocomposites made of a conductive polymer and a thermosensitive hydrogel: Strong effect of the preparation procedure on the properties. Polymer 2015, 78, 94–103. [Google Scholar] [CrossRef]
- Rivero, R.E.; Alustiza, F.; Rodríguez, N.; Bosch, P.; Miras, M.C.; Rivarola, C.R.; Barbero, C.A. Effect of functional groups on physicochemical and mechanical behavior of biocompatible macroporous hydrogels. React. Funct. Polym. 2015, 97, 77–85. [Google Scholar] [CrossRef]
- Alustiza, F.; Bellingeri, R.; Picco, N.; Motta, C.; Grosso, M.C.; Barbero, C.A.; Acevedo, D.F.; Vivas, A. IgY against enterotoxigenic Escherichia coli administered by hydrogel-carbon nanotubes composites to prevent neonatal diarrhoea in experimentally challenged piglets. Vaccine 2016, 34, 3291–3297. [Google Scholar] [CrossRef]
- Mulko, L.; Rivarola, C.R.; Barbero, C.A.; Acevedo, D.F. Bioethanol production by reusable Saccharomyces cerevisiae immobilized in a macroporous monolithic hydrogel matrices. J. Biotechnol. 2016, 233, 56–65. [Google Scholar] [CrossRef]
- Martinez, M.V.; Rodriguez, R.C.; Moncada, A.B.; Rivarola, C.R.; Bruno, M.M.; Miras, M.C.; Barbero, C.A. Electrochemistry of Tris(1,10-phenanthroline) iron (II) inside a polymeric hydrogel. Coupled chemical reactions and migration effects. J. Solid State Chem. 2016, 20, 2951–2960. [Google Scholar] [CrossRef]
- Martinez, M.V.; Bruno, M.M.; Miras, M.C.; Barbero, C.A. Electroactive polymers made by loading redox ions inside crosslinked polymeric hydrogels. Effects of hydrophobic interactions and solvent dynamics. Electrochim. Acta 2016, 219, 363–376. [Google Scholar] [CrossRef]
- Mulko, L.; Yslas, E.; Abel, S.B.; Rivarola, C.; Barbero, C.; Acevedo, D. Smart hydrogels: Application in bioethanol production. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Wiley: New York, NY, USA, 2017; Volume 1, pp. 79–105. [Google Scholar] [CrossRef]
- Martínez, M.V.; Rivarola, C.R.; Miras, M.C.; Barbero, C.A. A colorimetric iron sensor based on the partition of phenanthroline complexes into polymeric hydrogels. Combinatorial synthesis and high throughput screening of the hydrogel matrix. Sens. Actuators B Chem. 2017, 241, 9–32. [Google Scholar] [CrossRef]
- Rivero, R.; Alustiza, F.; Capella, V.; Liaudat, C.; Rodriguez, N.; Bosch, P.; Barbero, C.; Rivarola, C. Physicochemical properties of ionic and non-ionic biocompatible hydrogels in water and cell culture conditions: Relation with type of morphologies of bovine fetal fibroblasts in contact with the surfaces. Colloids Surf. B Biointerfaces 2017, 158, 488–497. [Google Scholar] [CrossRef]
- Pereyra, J.Y.; Cuello, E.A.; Rodriguez, R.C.; Barbero, C.A.; Yslas, E.I.; Salavagione, H.J.; Acevedo, D.F. Synthesis and characterization of GO-hydrogels composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 258, p. 012002. [Google Scholar] [CrossRef]
- Monerris, M.; Broglia, M.; Yslas, I.; Barbero, C.; Rivarola, C. Antibacterial polymeric nanocomposites synthesized by in-situ photoreduction of silver ions without additives inside biocompatible hydrogel matrices based on N-isopropylacrylamide and derivatives. Express Polym. Lett. 2017, 11, 946–962. [Google Scholar] [CrossRef]
- Martinez, M.V.; Molina, M.A.; Abel, S.B.; Barbero, C.A. Large Swelling Capacities of Crosslinked Poly(N-isopropylacrylamide) Gels in Organic Solvents. MRS Adv. 2018, 3, 3735–3740. [Google Scholar] [CrossRef]
- Martinez, M.V.; Rodriguez, R.C.; Bruno, M.M.; Acevedo, D.F.; Barbero, C.A. Simple electrochemical detection method employing a hydrogel soft matrix: Application in tap water. J. Electrochem. Soc. 2018, 165, H1021–H1027. [Google Scholar] [CrossRef]
- Bellingeri, R.; Mulko, L.; Molina, M.; Picco, N.; Alustiza, F.; Grosso, C.; Vivas, A.; Acevedo, D.F.; Barbero, C.A. Nanocomposites based on pH-sensitive hydrogels and chitosan decorated carbon nanotubes with antibacterial properties. Mater. Sci. Eng. C 2018, 90, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.V.; Molina, M.; Barbero, C.A. Poly(N-isopropylacrylamide) Cross-Linked Gels as Intrinsic Amphiphilic Materials: Swelling Properties Used to Build Novel Interphases. J. Phys. Chem. B 2018, 122, 9038–9048. [Google Scholar] [CrossRef] [PubMed]
- Broglia, M.F.; Balmaceda, I.; Carrizo, F.; Barbero, C.A.; Rivarola, C.R. Acid hydrogel matrixes as reducing/stabilizing agent for the in-situ synthesis of Ag-nanocomposites by UV irradiation: pH effect. Mater. Res. Express 2019, 6, 055021. [Google Scholar] [CrossRef]
- Pereyra, J.; Martinez, M.V.; Barbero, C.; Bruno, M.; Acevedo, D. Hydrogel-graphene oxide nanocomposites as electrochemical platform to simultaneously determine dopamine in presence of ascorbic acid using an unmodified glassy carbon electrode. J. Compos. Sci. 2019, 3, 1. [Google Scholar] [CrossRef]
- Monerris, M.; Broglia, M.F.; Yslas, E.I.; Barbero, C.A.; Rivarola, C.R. Highly effective antimicrobial nanocomposites based on hydrogel matrix and silver nanoparticles: Long-lasting bactericidal and bacteriostatic effects. Soft Matter 2019, 15, 8059–8066. [Google Scholar] [CrossRef]
- Mulko, L.; Pereyra, J.Y.; Rivarola, C.R.; Barbero, C.A.; Acevedo, D.F. Improving the retention and reusability of Alpha-amylase by immobilization in nanoporous polyacrylamide-graphene oxide nanocomposites. Int. J. Biol. Macromol. 2019, 122, 1253–1261. [Google Scholar] [CrossRef]
- Capella, V.; Rivero, R.E.; Liaudat, A.C.; Ibarra, L.E.; Roma, D.A.; Alustiza, F.; Mañas, F.; Barbero, C.A.; Bosch, P.; Rivarola, C.R.; et al. Cytotoxicity and bioadhesive properties of poly-N-isopropylacrylamide hydrogel. Heliyon 2019, 5, e01474. [Google Scholar] [CrossRef]
- Abel, S.B.; Riberi, K.; Rivarola, C.R.; Molina, M.; Barbero, C.A. Synthesis of a smart conductive block copolymer responsive to heat and near infrared light. Polymers 2019, 11, 1744. [Google Scholar] [CrossRef]
- Rivero, R.E.; Capella, V.; Liaudat, A.C.; Bosch, P.; Barbero, C.A.; Rodríguez, N.; Rivarola, C.R. Mechanical and physicochemical behavior of a 3D hydrogel scaffold during cell growth and proliferation. RSC Adv. 2020, 10, 5827–5837. [Google Scholar] [CrossRef]
- Riberi, K.; Abel, S.B.; Martinez, M.V.; Molina, M.A.; Rivarola, C.R.; Acevedo, D.F.; Rivero, R.; Cuello, E.A.; Gramaglia, R.; Barbero, C.A. Smart thermomechanochemical composite materials driven by different forms of electromagnetic radiation. J. Compos. Sci. 2020, 4, 3. [Google Scholar] [CrossRef]
- Abel, S.B.; Rivarola, C.R.; Barbero, C.A.; Molina, M. Electromagnetic radiation driving of volume changes in nanocomposites made of a thermosensitive hydrogel polymerized around conducting polymer nanoparticles. RSC Adv. 2020, 10, 9155–9164. [Google Scholar] [CrossRef]
- Mulko, L.E.; Cuello, E.A.; Barbero, C.A.; Pino, G.A.; Molina, M.; Rossa, M. Remote radiofrequency triggering of topography changes in a surface micropatterned PANI@PNIPAM nanocomposite. Appl. Surf. Sci. 2020, 509, 145370. [Google Scholar] [CrossRef]
- Casadey, R.; Broglia, M.; Barbero, C.; Criado, S.; Rivarola, C. Controlled release systems of natural phenolic antioxidants encapsulated inside biocompatible hydrogels. React. Funct. Polym. 2020, 156, 104729. [Google Scholar] [CrossRef]
- Abel, S.B.; Molina, M.; Rivarola, C.R.; Barbero, C.A. Pickering emulsions stabilized with PANI-NP. Study of the thermoresponsive behavior under heating and radiofrequency irradiation. J. Appl. Polym. Sci. 2021, 138, 50625. [Google Scholar] [CrossRef]
- Blois, D.A.; Liaudat, A.C.; Capella, V.; Morilla, G.; Rivero, R.E.; Broglia, M.F.; Barbero, C.A.; Rodríguez, N.; Bosch, P.; Rivarola, C.R. Interaction between Hyaluronic Acid Semi-Interpenetrated Hydrogel with Bull Spermatozoa: Studies of Sperm Attachment–Release and Sperm Quality. Adv. Mater. Interfaces 2021, 8, 2101155. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Martinez, M.V.; Bruno, M.M.; Barbero, C.A.; Abraham, G.A.; Acevedo, D.F. A modular platform based on electrospun carbon nanofibers and poly(N-isopropylacrylamide) hydrogel for sensor applications. Polym. Adv. Technol. 2021, 32, 4815–4825. [Google Scholar] [CrossRef]
- Liaudat, A.C.; Blois, D.; Capella, V.; Morilla, G.; Rivero, R.; Barbero, C.; Rodríguez, N.; Rivarola, C.; Bosch, P. Bull sperm selection by attachment to hyaluronic acid semi-interpenetrated hydrogels. Reprod. Domest. Anim. 2022, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.B.; Molina, M.A.; Rivarola, C.R.; Kogan, M.J.; Barbero, C.A. Smart polyaniline nanoparticles with thermal and photothermal sensitivity. Nanotechnology 2014, 25, 495602. [Google Scholar] [CrossRef]
- Cavallo, P.C.; Muñoz, D.J.; Miras, M.C.; Barbero, C.; Acevedo, D.F. Extracting kinetic parameters of aniline polymerization from thermal data of a batch reactor. Simulation of the thermal behavior of a reactor. J. Appl. Polym. Sci. 2014, 131, 39409. [Google Scholar] [CrossRef]
- Klomsiri, C.; Karplus, P.A.; Poole, L.B. Cysteine-based redox switches in enzymes. Antioxid. Redox Signal. 2011, 15, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, I.C.; Bueno, C.; Lissi, E.A.; Encinas, M.V. Thiols as chain transfer agents in free radical polymerization in aqueous solution. Polymer 2003, 44, 5559–5561. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.-M. Synthesis and Characterization of Modified Poly (vinyl alcohol) Membrane and Study of Its Enhanced Water-Induced Shape-Memory Behavior. J. Polym. Environ. 2022, 30, 3409–3419. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Cuello, E.A.; Mulko, L.E.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Development of micropatterning polyimide films for enhanced antifouling and antibacterial properties. Colloids Surf. B Biointerfaces 2020, 188, 110801. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, L.; Chen, C.; Yu, H.; Bai, H.; Wang, L.; Qin, M.; Feng, Y.; Feng, W. Liquid metal-created macroporous composite hydrogels with self-healing ability and multiple sensations as artificial flexible sensors. J. Mater. Chem. A 2021, 9, 875–883. [Google Scholar] [CrossRef]
- Acevedo, D.F.; Martínez, G.; Arana, J.T.; Yslas, E.I.; Mucklich, F.; Barbero, C.; Salavagione, H.J. Easy way to fabricate nanostructures on a reactive polymer surface. J. Phys. Chem. B 2009, 113, 14661–14666. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef]
- Molina, M.A. Development of Nano-Composites Based on Smart Hydrogels and Nano-Objects. Ph.D. Thesis, National University of Rio Cuarto, Rio Cuarto, Argentina, 1 May 2007. [Google Scholar]
- Martinez, M.V. Development of Polymeric Nanomaterials with Specific Solute Retention. Ph.D. Thesis, National University of Rio Cuarto, Rio Cuarto, Argentina, 23 July 2015. [Google Scholar]
- Balach, J.; Bruno, M.M.; Cotella, N.G.; Acevedo, D.F.; Barbero, C.A. Electrostatic self-assembly of hierarchical porous carbon microparticles. J. Power Source 2012, 199, 386–394. [Google Scholar] [CrossRef]
- Decher, G.; Eckle, M.; Schmitt, J.; Struth, B. Layer-by-layer assembled multicomposite films. Curr. Opin. Colloid Interface Sci. 1998, 3, 32–39. [Google Scholar] [CrossRef]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.-E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front. Bioeng. Biotechnol. 2020, 8, 654. [Google Scholar] [CrossRef]
- Pollack, G.H. Cells, Gels and the Engines of Life; Ebner and Sons Publishers: New York, NY, USA, 2001. [Google Scholar]
- Caykara, T.; Bulut, M.; Dilsiz, N.; Akyüz, Y. Macroporous poly(acrylamide) hydrogels: Swelling and shrinking behaviors. J. Macromol. Sci. A 2006, 43, 889–897. [Google Scholar] [CrossRef]
- Da Silva, L.B.J.; Oréfice, R.L. Synthesis and electromechanical actuation of a temperature, pH, and electrically responsive hydrogel. J. Polym. Res. 2014, 21, 466. [Google Scholar] [CrossRef]
- Torres, M.L.; Oberti, T.G.; Fernández, J.M. HEMA and alginate-based chondrogenic semi-interpenetrated hydrogels: Synthesis and biological characterization. J. Biomater. Sci. Polym. Ed. 2021, 32, 504–523. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wang, J.; Yang, S.; Wang, S. PSf/PANI nanocomposite membrane prepared by in situ blending of PSf and PANI/NMP. J. Membr. Sci. 2011, 376, 83–95. [Google Scholar] [CrossRef]
- Hüther, A.; Xu, X.; Maurer, G. Swelling of n-isopropyl acrylamide hydrogels in water and aqueous solutions of ethanol and acetone. Fluid Phase Equilib. 2004, 219, 231–244. [Google Scholar] [CrossRef]
- Orlov, Y.; Xu, X.; Maurer, G. Equilibrium swelling of N-isopropyl acrylamide based ionic hydrogels in aqueous solutions of organic solvents: Comparison of experiment with theory. Fluid Phase Equilib. 2006, 249, 6–16. [Google Scholar] [CrossRef]
- Orlov, Y.; Xu, X.; Maurer, G. Swelling of nonionic N-isopropyl acrylamide hydrogels in aqueous solutions of (acetic acid or pyridine). Fluid Phase Equilib. 2005, 238, 87–94. [Google Scholar] [CrossRef]
- Althans, D.; Langenbach, K.; Enders, S. Influence of different alcohols on the swelling behaviour of hydrogels. Mol. Phys. 2012, 110, 1391–1402. [Google Scholar] [CrossRef]
- Wu, S.; Shanks, R.A. Solubility study of polyacrylamide in polar solvents. J. Appl. Polym. Sci. 2004, 93, 1493–1499. [Google Scholar] [CrossRef]
- Narváez, W.E.V.; Jiménez, E.I.; Hernández-Rodríguez, M.; Rocha-Rinza, T. Simple method to estimate relative hydrogen bond basicities of amides and imides in chloroform. J. Mol. Struct. 2018, 1173, 608–611. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Akbar, F.; Ahmad, G.; Najeeb, J.; Hussain, Z.U.N. Recent progress of poly(N-isopropylacrylamide) hybrid hydrogels: Synthesis, fundamentals and applications—Review. Soft Mater. 2018, 16, 228–247. [Google Scholar] [CrossRef]
- Adelson, P.D. Hypothermia following Pediatric Traumatic Brain Injury. J. Neurotrauma 2009, 26, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Planes, G.A.; Miras, M.C.; Barbero, C. Strong effects of counterions on the electrochemistry of poly(N-methylaniline) thin films. Polym. Int. 2002, 51, 429–433. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Lira, L.M.; de Torresi, S.I.C. Conducting polymer–hydrogel composites for electrochemical release devices: Synthesis and characterization of semi-interpenetrating polyaniline–polyacrylamide networks. Electrochem. Commun. 2005, 7, 717–723. [Google Scholar] [CrossRef]
- Marcus, R.A. On the Theory of Electron-Transfer Reaction VI. Unified Treatment of Homogeneous and Electrode Reactions. J. Chem. Phys. 1965, 43, 679. [Google Scholar] [CrossRef]
- Muya, F.N.; Sunday, C.E.; Baker, P.; Iwuoha, E. Environmental remediation of heavy metal ions from aqueous solution through hydrogel adsorption: A critical review. Water Sci. Technol. 2016, 73, 983–992. [Google Scholar] [CrossRef]
- Grygolowicz-Pawlak, E.; Crespo, G.A.; Afshar, M.G.; Mistlberger, G.; Bakker, E. Potentiometric Sensors with Ion-Exchange Donnan Exclusion Membranes. Anal. Chem. 2013, 85, 6208–6212. [Google Scholar] [CrossRef]
- Available online: http://www.water-esearch.net/index.php/standards/secondary-standards (accessed on 22 June 2022).
- Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31998L0083 (accessed on 22 June 2022).
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. In Vitro 2013, 27, 330–338. [Google Scholar] [CrossRef]
- Share, H.; Ali, M.; Asl, H.; Khajenoori, M. Experimental measurement and correlation of solubility of β-carotene in pure and ethanol-modified subcritical water, Chinese. J. Chem. Eng. 2020, 28, 2620–2625. [Google Scholar] [CrossRef]
- Masuda, H.; Tanaka, S.; Kaeriyama, K. Soluble conducting polypyrrole: Poly(3-octylpyrrole). J. Chem. Soc. Chem. Commun. 1989, 11, 725–726. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Wang, X.; Feng, Q.; Pei, M.; Zhang, G. Synthesis and characterization of three novel conjugated polythiophene derivatives. Des. Monomers. Polym. 2013, 16, 339–348. [Google Scholar] [CrossRef]
- Lanzi, M.; Salatelli, E.; Giorgini, L.; Mucci, A.; Pierini, F.; Di-Nicola, F.P. Water-soluble polythiophenes as efficient charge-transport layers for the improvement of photovoltaic performance in bulk heterojunction polymeric solar cells. Eur. Polym. J. 2017, 97, 378–388. [Google Scholar] [CrossRef]
- Barbero, C.; Kötz, R. Electrochemical formation of a self-doped conductive polymer in the absence of a supporting electrolyte. The copolymerization of o-aminobenzenesulfonic acid and aniline. Adv. Mater. 1994, 6, 577–580. [Google Scholar] [CrossRef]
- Ono, T.; Ohta, M.; Sada, K. Ionic polymers act as polyelectrolytes in nonpolar media. ACS Macro Lett. 2012, 1, 1270–1273. [Google Scholar] [CrossRef]
- Sun, X.-G.; Liu, G.; Xie, J.; Han, Y.; Kerr, J.B. New gel polyelectrolytes for rechargeable lithium batteries. Solid State Ion 2004, 175, 713–716. [Google Scholar] [CrossRef]
- Caykara, T.; Izol, D.; Birlik, G.; Akaoǧlu, B. Adsorption of surfactant by hydrophobically modified Poly [2-(diethylamino) ethylmethacrylate-co-N-vinyl-2-pyrrolidone/octadecyl acrylate] hydrogels and effect of surfactant adsorption on the volume phase transition. J. Appl. Polym. Sci. 2007, 103, 3771–3775. [Google Scholar] [CrossRef]
- Ono, T.; Sada, K. Toward the design of superabsorbent materials for non-polar organic solvents and oils: Ionic content dependent swelling behaviour of cross-linked poly (octadecyl acrylate)-based lipophilic polyelectrolytes. J. Mater. Chem. 2012, 22, 20962–20967. [Google Scholar] [CrossRef]
- Shimamoto, G.G.; Aricetti, J.A.; Tubino, M. A Simple, Fast, and green titrimetric method for the determination of the iodine value of vegetable oils without wijs solution (ICl). Food Anal. Methods 2016, 9, 2479–2483. [Google Scholar] [CrossRef]
- Gesser, H.D.; Fu, S. Removal of Aldehydes and Acidic Pollutants from Indoor Air. Environ. Sci. Technol. 1990, 24, 495–497. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, S.; Jiang, S.; Xiao, F.; Deng, G.-J. Electrosynthesis of azobenzenes directly from nitrobenzenes. Chin. J. Chem. 2021, 39, 3334–3338. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, D.; Chen, H.; Zhang, Y.; Liu, Y.; Ren, B.; Zheng, J. A multiscale polymerization framework towards network structure and fracture of double-network hydrogels. Npj Comput. Mater. 2021, 7, 39. [Google Scholar] [CrossRef]
- Dharmasiri, M.B.; Mudiyanselage, T.K. Thermo-responsive poly (N-isopropyl acrylamide) hydrogel with increased response rate. Polym. Bull. 2021, 78, 3183–3198. [Google Scholar] [CrossRef]
- Pei, Y.; Zhao, L.; Du, G.; Li, N.; Xu, K.; Yang, H. Investigation of the degradation and stability of acrylamide-based polymers in acid solution: Functional monomer modified polyacrylamide. Petroleum 2016, 2, 399–407. [Google Scholar] [CrossRef]
- Weißenborn, E.; Braunschweig, B. Hydroxypropyl cellulose as a green polymer for thermo-responsive aqueous foams. Soft Matter 2019, 15, 2876–2883. [Google Scholar] [CrossRef]
- Abel, S.B. Synergistic Nanocomposites Based on Thermosensitive and Conductive Polymers. Ph.D. Thesis, National University of Rio Cuarto, Rio Cuarto, Argentina, 23 February 2018. [Google Scholar]
- Salemis, P.; Rinaudo, M. Molecular weight-viscosity relationship for amylopectin, a highly branched polymer. Polym. Bull. 1984, 12, 283–285. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, L.; Ren, M.-H.; BeMiller, J.N. Developments in Hydroxypropylation of Starch: A Review. Starch 2019, 71, 1800167. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Fernández, M.; Sinisterra, J.V.; Alcántara, A.R. 2-Methyltetrahydrofuran as a suitable green solvent for phthalimide functionalization promoted by supported KF. Green Chem. 2010, 12, 1380–1382. [Google Scholar] [CrossRef]









| Structures | Fabrication Method | Applications | Refs |
|---|---|---|---|
| Nanoporous gel | Radical polymerization with cross-linkers | Thermosensitive gels Cell growth scaffolds Amphiphilic gels Electrochemical sensors | [7,10,11,15,30,31,33,38] |
| s-IPN | Absorption of true polymer solution | Photothermomechanical actuators Pressure sensors | [19] |
| Macroporous gel | Cryogelation | Biological cell scaffold Absorption of nanoparticles | [12,20,22] |
| Macroporous nanocomposite | Swelling the macroporous gel in a dispersion of the nanomaterial. | Photothermomechanical actuators | [12] |
| Microparticles | Radical polymerization inside water in oil emulsions | Self-assembled monolayers with cell-like fusion | [60] |
| Nanoparticles | Controlled nucleation and growth polymerization with charged comonomer (e.g., AMPS) | Thermosensitive dispersions | [60,61] |
| Nanocomposites | In situ formation of nanomaterial (metal, CP) inside the gel | Bactericide (Ag NPs) Pressure sensor (CP) | [13,29,36,43] |
| Nanocomposites | Formation of gel around the nanomaterial (CP nanoparticles, graphene) | Pressure sensors Photothermal bactericide films Protective carrier of bioactive compounds | [17,18] |
| Composition (% in Feed) | Nanoporous | Macroporous | |||
|---|---|---|---|---|---|
| NIPAM | Comonomer | wnf (%) | wf (%) | wnf (%) | wf (%) |
| 100 | 0 | 34.5 | 62.9 | 18.5 | 76.9 |
| 90 | HMA (10%) | 60.3 | 30.5 | 50.6 | 45.4 |
| 98 | AMPS (2%) | 55.1 | 42.7 | 2.4 | 94.7 |
| Composition (% in Feed) | Elasticity Modulus (kPa) | |||
|---|---|---|---|---|
| NIPAM | Comonomer | Nanoporous | Macroporous (Collinear) | Macroporous (Transversal) |
| 100 | 0 | 5.9 | 33.3 | 10.9 |
| 90 | HMA (10%) | 5.9 | 3.1 | 3.1 |
| 98 | AMPS (2%) | 6.6 | 20.5 | 12.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, C.A.; Martínez, M.V.; Acevedo, D.F.; Molina, M.A.; Rivarola, C.R. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol 2022, 2, 440-475. https://doi.org/10.3390/macromol2030028
Barbero CA, Martínez MV, Acevedo DF, Molina MA, Rivarola CR. Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol. 2022; 2(3):440-475. https://doi.org/10.3390/macromol2030028
Chicago/Turabian StyleBarbero, Cesar A., María V. Martínez, Diego F. Acevedo, María A. Molina, and Claudia R. Rivarola. 2022. "Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications" Macromol 2, no. 3: 440-475. https://doi.org/10.3390/macromol2030028
APA StyleBarbero, C. A., Martínez, M. V., Acevedo, D. F., Molina, M. A., & Rivarola, C. R. (2022). Cross-Linked Polymeric Gels and Nanocomposites: New Materials and Phenomena Enabling Technological Applications. Macromol, 2(3), 440-475. https://doi.org/10.3390/macromol2030028