Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes
Abstract
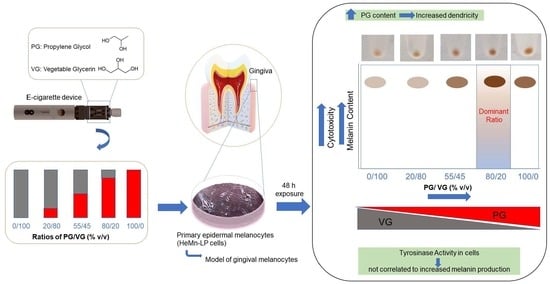
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Estimation of Cellular Melanin Content
2.5. Microscopic Observation
2.6. Intracellular Tyrosinase Activity
2.7. Statistical Analysis
3. Results
3.1. Effect of E-Liquids on Cell Viability
3.2. Effect of E-Liquids on Cellular Melanin Content
3.3. Effect of E-Liquids on Cellular Morphology
3.4. Effect of E-Liquids on Cellular Tyrosinase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, C.J.; Cheng, J.M. Electronic cigarettes: Product characterisation and design considerations. Tob. Control. 2014, 23, ii4–ii10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, D.T.; Borland, R.; Lindblom, E.N.; Goniewicz, M.L.; Meza, R.; Holford, T.R.; Yuan, Z.; Luo, Y.; O’Connor, R.J.; Niaura, R. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob. Control. 2018, 27, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratton, K.; Kwan, L.Y.; Eaton, D.L. Public Health Consequences of E-Cigarettes: Consensus Study Report; National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Farsalinos, K.E.; Polosa, R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Saf. 2014, 5, 67–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, I.; Sandeep, S.; Rodriguez, J. The oral health impact of electronic cigarette use: A systematic review. Crit. Rev. Toxicol. 2020, 50, 97–127. [Google Scholar] [CrossRef]
- Szumilas, P.; Wilk, A.; Szumilas, K.; Karakiewicz, B. The Effects of E-Cigarette Aerosol on Oral Cavity Cells and Tissues: A Narrative Review. Toxics 2022, 10, 74. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Schneider, A.; Fox, S.C.; Meyer, M.; Meboldt, M.; Attin, T.; Schmidlin, P.R. Cytotoxic and Inflammatory Effects of Electronic and Traditional Cigarettes on Oral Gingival Cells Using a Novel Automated Smoking Instrument: An In Vitro Study. Toxics 2022, 10, 179. [Google Scholar] [CrossRef]
- Alanazi, H.; Rouabhia, M. Effect of e-cigarette aerosol on gingival mucosa structure and proinflammatory cytokine response. Toxicol. Rep. 2022, 9, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Wadia, R.; Booth, V.; Yap, H.; Moyes, D. A pilot study of the gingival response when smokers switch from smoking to vaping. Br. Dent. J. 2016, 221, 722–726. [Google Scholar] [CrossRef]
- Harvanko, A.; Kryscio, R.; Martin, C.; Kelly, T. Stimulus effects of propylene glycol and vegetable glycerin in electronic cigarette liquids. Drug Alcohol Depend. 2019, 194, 326–329. [Google Scholar] [CrossRef]
- Smith, T.T.; Heckman, B.W.; Wahlquist, A.E.; Cummings, K.M.; Carpenter, M.J. The impact of e-liquid propylene glycol and vegetable glycerin ratio on ratings of subjective effects, reinforcement value, and use in current smokers. Nicotine Tob. Res. 2020, 22, 791–797. [Google Scholar] [CrossRef]
- Clapp, P.W.; Jaspers, I. Electronic cigarettes: Their constituents and potential links to asthma. Curr. Allergy Asthma Rep. 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Baassiri, M.; Talih, S.; Salman, R.; Karaoghlanian, N.; Saleh, R.; El Hage, R.; Saliba, N.; Shihadeh, A. Clouds and “throat hit”: Effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols. Aerosol Sci. Technol. 2017, 51, 1231–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spindle, T.R.; Talih, S.; Hiler, M.M.; Karaoghlanian, N.; Halquist, M.S.; Breland, A.B.; Shihadeh, A.; Eissenberg, T. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 2018, 188, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talih, S.; Salman, R.; El-Hage, R.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.; Shihadeh, A. Effect of free-base and protonated nicotine on nicotine yield from electronic cigarettes with varying power and liquid vehicle. Sci. Rep. 2020, 10, 16263. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.; Coblentz, T.; Bruce, J.; Nassabeh, S.; Plants, R.; Burrage, E.; Mills, A.; Chantler, P.; Olfert, I. Effect of E-liquid Base Solution (Vegetable Glycerin or Propylene Glycol) on Aortic Function in Mice. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Morshed, K.M.; Jain, S.K.; McMartin, K.E. Propylene glycol-mediated cell injury in a primary culture of human proximal tubule cells. Toxicol. Sci. 1998, 46, 410–417. [Google Scholar] [CrossRef]
- Doi, A.M.; Roycroft, J.H.; Herbert, R.A.; Haseman, J.K.; Hailey, J.R.; Chou, B.J.; Dill, J.A.; Grumbein, S.L.; Miller, R.A.; Renne, R.A. Inhalation toxicology and carcinogenesis studies of propylene glycol mono-t-butyl ether in rats and mice. Toxicology 2004, 199, 1–22. [Google Scholar] [CrossRef]
- Yaucher, N.E.; Fish, J.T.; Smith, H.W.; Wells, J.A. Propylene glycol-associated renal toxicity from lorazepam infusion. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 1094–1099. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Jabłczyńska, K.; Odziomek, M.; Schlage, W.K.; Kuczaj, A.K. Physicochemical studies of direct interactions between lung surfactant and components of electronic cigarettes liquid mixtures. Inhal. Toxicol. 2018, 30, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Hayeck, N.; Zoghzoghi, C.; Karam, E.; Salman, R.; Karaoghlanian, N.; Shihadeh, A.; Eissenberg, T.; Zein El Dine, S.; Saliba, N.A. Carrier solvents of electronic nicotine delivery systems alter pulmonary surfactant. Chem. Res. Toxicol. 2021, 34, 1572–1577. [Google Scholar] [CrossRef]
- Beklen, A.; Uckan, D. Electronic cigarette liquid substances propylene glycol and vegetable glycerin induce an inflammatory response in gingival epithelial cells. Hum. Exp. Toxicol. 2021, 40, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Chung, S.; Dennis, J.; Yoshida, M.; Aguiar, C.; Aller, S.; Mendes, E.; Schmid, A.; Sabater, J.; Baumlin, N. Vegetable glycerin e-cigarette aerosols cause airway inflammation and ion channel dysfunction. Front. Pharmacol. 2022, 13, 1012723. [Google Scholar] [CrossRef]
- Natesan, S.C.; Ramakrishnan, B.P.; Krishnapillai, R.; Thomas, P. Biophysiology of oral mucosal melanocytes. J. Health Sci. 2019, 2, 47–51. [Google Scholar] [CrossRef]
- Feller, L.; Masilana, A.; Khammissa, R.A.; Altini, M.; Jadwat, Y.; Lemmer, J. Melanin: The biophysiology of oral melanocytes and physiological oral pigmentation. Head Face Med. 2014, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Suryaningsih, B.E. Melanogenesis and its associated signalings. Bali Med. J. 2020, 9, 327–331. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Iozumi, K.; Hoganson, G.E.; Pennella, R.; Everett, M.A.; Fuller, B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993, 100, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Sanadi, R.M.; Deshmukh, R.S. Expression of tyrosinase gene in gingiva: A pilot study. J. Oral Maxillofac. Pathol. 2022, 26, 422. [Google Scholar]
- Moneim, R.A.A.; El Deeb, M.; Rabea, A.A. Gingival pigmentation (cause, treatment and histological preview). Future Dent. J. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Firoozi, P.; Noormohammadi, R.; Rafieyan, S. Effect of environmental tobacco smoke on oral pigmentation: A systematic review. J. Oral Health Oral Epidemiol. 2020, 9, 1–6. [Google Scholar]
- Rotbeh, A.; Kazeminia, M.; Rajati, F. Full title: Global prevalence of oral pigmentation and its related factors: A systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e411–e424. [Google Scholar] [CrossRef] [PubMed]
- Tadakamadla, J.; Kumar, S.; Nagori, A.; Tibdewal, H.; Duraiswamy, P.; Kulkarni, S. Effect of smoking on oral pigmentation and its relationship with periodontal status. Dent. Res. J. 2012, 9, S112. [Google Scholar]
- Bardellini, E.; Amadori, F.; Conti, G.; Majorana, A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol. Scand. 2018, 76, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Murata, K.; Ushio, K.; Sakai, R. Dose-response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch. Environ. Health Int. J. 1983, 38, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Peace, M.R.; Baird, T.R.; Smith, N.; Wolf, C.E.; Poklis, J.L.; Poklis, A. Concentration of nicotine and glycols in 27 electronic cigarette formulations. J. Anal. Toxicol. 2016, 40, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Lee, E.S.; Nguyen, C.; Zhu, Y. Effects of propylene glycol, vegetable glycerin, and nicotine on emissions and dynamics of electronic cigarette aerosols. Aerosol Sci. Technol. 2020, 54, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Eversole, A.; Crabtree, M.; Spindle, T.R.; Baassiri, M.; Eissenberg, T.; Breland, A. E-cigarette Solvent Ratio and Device Power Influence Ambient Air Particulate Matter. Tob. Regul. Sci. 2021, 7, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment. Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Depigmenting effect of Xanthohumol from hop extract in MNT-1 human melanoma cells and normal human melanocytes. Biochem. Biophys. Rep. 2021, 26, 100955. [Google Scholar] [CrossRef] [PubMed]
- Health, U.D.o.; Services, H. E-cigarette use among youth and young adults: A report of the Surgeon General. JAMA Pediatr. 2016, 171, 209–210. [Google Scholar]
- Fruet-Arruda, R.T.; Anselmo, G.G.; Tortamano, A.C.A.; Rossi, A.L.; Biffi, M.B.; Marco, R.L.; Kato, I.T.; Nuñez, S.C.; Prates, R.A. Melanin pigmented gingival tissue impairs red-light lateral scattering for antimicrobial photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102135. [Google Scholar] [CrossRef]
- Syaify, A. Depigmentation of Gingival Smoker’s Melanosis Using Scalpel Surgical Technique: A Case Report. KnE Med. 2022, 270–281. [Google Scholar] [CrossRef]
- Prasad, D.; Sunil, S.; Mishra, R. Treatment of gingival pigmentation: A case series. Indian J. Dent. Res. 2005, 16, 171. [Google Scholar]
- Mokeem, S.A. Management of gingival hyperpigmentation by surgical abrasion: Report of three cases. Saudi Dent. J. 2006, 18, 162–166. [Google Scholar]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739. [Google Scholar] [PubMed]
- Pai, A.; Prasad, S.; Patil, B.A.; Dyasanoor, S.; Hedge, S. Oral pigmentation: Case report and review of malignant melanoma with flow charts for diagnosis and treatment. Gen. Dent. 2012, 60, 410–416; quiz 417. [Google Scholar] [PubMed]
- Eisen, D. Disorders of pigmentation in the oral cavity. Clin. Dermatol. 2000, 18, 579–587. [Google Scholar] [CrossRef]
- Nilima, S.; Vandana, K. Melanin: A scavenger in gingival inflammation. Indian J. Dent. Res. 2011, 22, 38. [Google Scholar]
- Goenka, S.; Simon, S.R. Effects of Fluoride Exposure on Primary Human Melanocytes from Dark and Light Skin. Toxics 2020, 8, 114. [Google Scholar] [CrossRef]
- Komura, M.; Sato, T.; Yoshikawa, H.; Nitta, N.A.; Suzuki, Y.; Koike, K.; Kodama, Y.; Seyama, K.; Takahashi, K. Propylene glycol, a component of electronic cigarette liquid, damages epithelial cells in human small airways. Respir. Res. 2022, 23, 216. [Google Scholar] [CrossRef]
- Sinha, I.; Goel, R.; Bitzer, Z.T.; Trushin, N.; Liao, J.; Sinha, R. Evaluating electronic cigarette cytotoxicity and inflammatory responses in vitro. Tob. Induc. Dis. 2022, 20, 1–13. [Google Scholar] [CrossRef]
- Leslie, L.J.; Vasanthi Bathrinarayanan, P.; Jackson, P.; Mabiala Ma Muanda, J.A.; Pallett, R.; Stillman, C.J.; Marshall, L.J. A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal. Toxicol. 2017, 29, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khachatoorian, C.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. E-cigarette fluids and aerosol residues cause oxidative stress and an inflammatory response in human keratinocytes and 3D skin models. Toxicol. Vitr. 2021, 77, 105234. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Marescotti, D.; Martin, F.; Scotti, E.; Guedj, E.; Acali, S.; Dulize, R.; Baumer, K.; Peric, D.; Frentzel, S. In vitro systems toxicology assessment of nonflavored e-cigarette liquids in primary lung epithelial cells. Appl. Vitr. Toxicol. 2017, 3, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Coakley, R.C.; Mascenik, T.; Rowell, T.R.; Davis, E.S.; Rogers, K.; Webster, M.J.; Dang, H.; Herring, L.E.; Sassano, M.F. Chronic e-cigarette exposure alters the human bronchial epithelial proteome. Am. J. Respir. Crit. Care Med. 2018, 198, 67–76. [Google Scholar] [CrossRef]
- Woodall, M.; Jacob, J.; Kalsi, K.; Schroeder, V.; Davis, E.; Kenyon, B.; Khan, I.; Garnett, J.; Tarran, R.; Baines, D. E-cigarette constituents propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L957–L967. [Google Scholar] [CrossRef]
- Ghosh, A.; Coakley, R.D.; Ghio, A.J.; Muhlebach, M.S.; Esther, C.R., Jr.; Alexis, N.E.; Tarran, R. Chronic e-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am. J. Respir. Crit. Care Med. 2019, 200, 1392–1401. [Google Scholar] [CrossRef]
- Bin, B.-H.; Bhin, J.; Yang, S.H.; Choi, D.-H.; Park, K.; Shin, D.W.; Lee, A.-Y.; Hwang, D.; Cho, E.-G.; Lee, T.R. Hyperosmotic stress reduces melanin production by altering melanosome formation. PLoS ONE 2014, 9, e105965. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A.; Ren, W.-Y.; Khorlin, A.; Lesiak, K.; Conklin, D.; Watanabe, K.A.; Seidman, M.M.; George, J. Aliphatic and alicyclic diols induce melanogenesis in cultured cells and guinea pig skin. J. Investig. Dermatol. 1998, 110, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. Molecular and biochemical basis of minocycline-induced Hyperpigmentation—The study on normal human melanocytes exposed to UVA and UVB radiation. Int. J. Mol. Sci. 2021, 22, 3755. [Google Scholar] [CrossRef]
- Wang, J.; Brown, I.; Goodarzi, H. Minocycline-Induced Gum Pigmentation during Treatment for Acne Vulgaris. Case Rep. Pediatr. 2022, 2022, 9493061. [Google Scholar] [CrossRef] [PubMed]
- Tosios, K.I.; Kalogirou, E.-M.; Sklavounou, A. Drug-associated hyperpigmentation of the oral mucosa: Report of four cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, e54–e66. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. Comparative study of curcumin and its hydrogenated metabolites, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin, on melanogenesis in B16F10 and MNT-1 cells. Cosmetics 2021, 8, 4. [Google Scholar] [CrossRef]
- Slominski, A. Coming of age of melanogenesis-related proteins. Arch. Pathol. Lab. Med. 2002, 126, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Jia, Q.; Tian, W.; Li, B.; Chen, W.; Zhang, W.; Xie, Y.; Cheng, N.; Chen, Q.; Xiao, J.; Zhang, Y. Transient Receptor Potential channels, TRPV1 and TRPA1 in melanocytes synergize UV-dependent and UV-independent melanogenesis. Br. J. Pharmacol. 2021, 178, 4646–4662. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Liu, Y.; Guo, H.; Li, Z.; Zou, W.; Liu, J.; Song, Z. TRPA1 Promotes UVB-Induced Skin Pigmentation by Regulating Melanosome Luminal pH. Exp. Dermatol. 2022. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wu, W.; Liu, Y.; Xiao, Y.; Qi, D.; Zhao, G.; Zhou, M.; Wang, H.; Liu, J. TRPA1 promotes melanosome phagocytosis in keratinocytes via PAR-2/CYLD axis. J. Dermatol. Sci. 2022, 106, 181–188. [Google Scholar] [CrossRef]
- Bellono, N.W.; Kammel, L.G.; Zimmerman, A.L.; Oancea, E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. USA 2013, 110, 2383–2388. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, E.; Hiyama, T.Y.; Noda, M. Osmosensitivity of transient receptor potential vanilloid 1 is synergistically enhanced by distinct activating stimuli such as temperature and protons. PLoS ONE 2011, 6, e22246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Chen, J.; Faltynek, C.R.; Moreland, R.B.; Neelands, T.R. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur. J. Neurosci. 2008, 27, 605–611. [Google Scholar] [CrossRef]
- Lessmann, H.; Schnuch, A.; Geier, J.; Uter, W. Skin-sensitizing and irritant properties of propylene glycol: Data analysis of a multicentre surveillance network (IVDK*) and review of the literature. Contact Dermat. 2005, 53, 247–259. [Google Scholar] [CrossRef]
- Jordt, S.; Jabba, S.; Ghoreshi, K.; Smith, G.; Morris, J. Propylene Glycol and Glycerin in E-Cigarettes Elicit Respiratory Irritation Responses and Modulate Human Sensory Irritant Receptor Function. In B107. Effects of E-Cigarettes and Their Components on Respiratory Dysfunction, Inflammation, and Repair; American Thoracic Society: New York, NY, USA, 2019; p. A4169. [Google Scholar]
- Niedermirtl, F.; Eberhardt, M.; Namer, B.; Leffler, A.; Nau, C.; Reeh, P.W.; Kistner, K. Etomidate and propylene glycol activate nociceptive TRP ion channels. Mol. Pain 2018, 14, 1744806918811699. [Google Scholar] [CrossRef]
- Bell, R.L.; McAuley, D.F.; Shyamsundar, M.; O’Kane, C.M.; Dombrowski, Y. E-cigarette vapour from base components propylene glycol and vegetable glycerine inhibits inflammatory response in macrophages and epithelial cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Goel, R.; Reilly, S.M.; Foulds, J.; Muscat, J.; Elias, R.J.; Richie, J.P., Jr. Effects of solvent and temperature on free radical formation in electronic cigarette aerosols. Chem. Res. Toxicol. 2018, 31, 4–12. [Google Scholar] [CrossRef]
- Kim, S.A.; Smith, S.; Beauchamp, C.; Song, Y.; Chiang, M.; Giuseppetti, A.; Frukhtbeyn, S.; Shaffer, I.; Wilhide, J.; Routkevitch, D. Cariogenic potential of sweet flavors in electronic-cigarette liquids. PLoS ONE 2018, 13, e0203717. [Google Scholar] [CrossRef] [Green Version]
- Schober, W.; Szendrei, K.; Matzen, W.; Osiander-Fuchs, H.; Heitmann, D.; Schettgen, T.; Jörres, R.A.; Fromme, H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 2014, 217, 628–637. [Google Scholar] [CrossRef]
- Nguyen, C.; Li, L.; Sen, C.A.; Ronquillo, E.; Zhu, Y. Fine and ultrafine particles concentrations in vape shops. Atmos. Environ. 2019, 211, 159–169. [Google Scholar] [CrossRef]
- Ingebrethsen, B.J.; Cole, S.K.; Alderman, S.L. Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol. 2012, 24, 976–984. [Google Scholar] [CrossRef]
- Yang, X.; Peng, F.; Huang, J.; Chen, Z.; Zhang, J. Particulate matter 2.5 induced hyperpigmentation in reconstructed human epidermis model (MelaKutis®). Chin. Med. J. 2022, 135, 502–504. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, E.J.; Luo, E.; Choi, J.; Kim, J.Y.; Kim, S.; Kim, S.-H.; Bae, Y.J.; Park, S.; Lee, J. Particulate Matter Promotes Melanin Production through Endoplasmic Reticulum Stress—Mediated IRE1α Signaling. J. Investig. Dermatol. 2022, 142, 1425–1434.e1426. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tsuji, G.; Zhang, J.-Z.; Chen, Z.; Furue, M. Potential role of PM2.5 in melanogenesis. Environ. Int. 2019, 132, 105063. [Google Scholar] [CrossRef] [PubMed]
- Duell, A.K.; Pankow, J.F.; Gillette, S.M.; Peyton, D.H. Boiling points of the propylene glycol+ glycerol system at 1 atmosphere pressure: 188.6–292 C without and with added water or nicotine. Chem. Eng. Commun. 2018, 205, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Kerber, P.J.; Duell, A.K.; Peyton, D.H. Ratio of Propylene Glycol to Glycerol in E-Cigarette Reservoirs Is Unchanged by Vaping as Determined by 1H NMR Spectroscopy. Chem. Res. Toxicol. 2021, 34, 1846–1849. [Google Scholar] [CrossRef]
- Jensen, R.P.; Strongin, R.M.; Peyton, D.H. Solvent chemistry in the electronic cigarette reaction vessel. Sci. Rep. 2017, 7, 42549. [Google Scholar] [CrossRef] [Green Version]
- Escobar, Y.-N.H.; Nipp, G.; Cui, T.; Petters, S.S.; Surratt, J.D.; Jaspers, I. In vitro toxicity and chemical characterization of aerosol derived from electronic cigarette humectants using a newly developed exposure system. Chem. Res. Toxicol. 2020, 33, 1677–1688. [Google Scholar] [CrossRef]
- Schneller, L.M.; Vanderbush, T.S.; O’Connor, R.J. Can Established Vapers Distinguish Different PG: VG Ratios? A Pilot Study. Tob. Regul. Sci. 2018, 4, 73. [Google Scholar] [CrossRef]
- Dalrymple, A.; Bean, E.-J.; Badrock, T.C.; Weidman, R.A.; Thissen, J.; Coburn, S.; Murphy, J. Enamel staining with e-cigarettes, tobacco heating products and modern oral nicotine products compared with cigarettes and snus: An in vitro study. Am. J. Dent. 2021, 34, 3–9. [Google Scholar]
- Dalrymple, A.; Badrock, T.C.; Terry, A.; Barber, M.; Hall, P.J.; Thorne, D.; Gaca, M.D.; Coburn, S.; Proctor, C. Assessment of enamel discoloration in vitro following exposure to cigarette smoke and emissions from novel vapor and tobacco heating products. Am. J. Dent. 2018, 31, 227–233. [Google Scholar]
- Behar, R.Z.; Wang, Y.; Talbot, P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control. 2018, 27, 325–333. [Google Scholar] [CrossRef]
- McAlinden, K.D.; Lu, W.; Ferdowsi, P.V.; Myers, S.; Markos, J.; Larby, J.; Chia, C.; Weber, H.C.; Haug, G.; Eapen, M.S. Electronic cigarette aerosol is cytotoxic and increases ACE2 expression on human airway epithelial cells: Implications for SARS-CoV-2 (COVID-19). J. Clin. Med. 2021, 10, 1028. [Google Scholar] [CrossRef]
- Tellez, C.S.; Juri, D.E.; Phillips, L.M.; Do, K.; Yingling, C.M.; Thomas, C.L.; Dye, W.W.; Wu, G.; Kishida, S.; Kiyono, T. Cytotoxicity and genotoxicity of E-cigarette generated aerosols containing diverse flavoring products and nicotine in oral epithelial cell lines. Toxicol. Sci. 2021, 179, 220–228. [Google Scholar] [CrossRef]
- Delijewski, M.; Beberok, A.; Otręba, M.; Wrześniok, D.; Rok, J.; Buszman, E. Effect of nicotine on melanogenesis and antioxidant status in HEMn-LP melanocytes. Environ. Res. 2014, 134, 309–314. [Google Scholar] [CrossRef]
- Feyerabend, C.; Higenbottam, T.; Russell, M. Nicotine concentrations in urine and saliva of smokers and non-smokers. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 1002–1004. [Google Scholar] [CrossRef] [Green Version]
- Yu, V.; Rahimy, M.; Korrapati, A.; Xuan, Y.; Zou, A.E.; Krishnan, A.R.; Tsui, T.; Aguilera, J.A.; Advani, S.; Crotty Alexander, L.E.; et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016, 52, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Bestman, E.G.; Brooks, J.K.; Mostoufi, B.; Bashirelahi, N. What every dentist needs to know about electronic cigarettes. Gen. Dent. 2021, 69, 31–35. [Google Scholar]
- Sultan, A.S.; Jessri, M.; Farah, C.S. Electronic nicotine delivery systems: Oral health implications and oral cancer risk. J. Oral Pathol. Med. 2021, 50, 316–322. [Google Scholar] [CrossRef]
- Smart, D.J.; Helbling, F.R.; McHugh, D.; Vanscheeuwijck, P. Baseline effects of non-flavored e-liquids in the in vitro micronucleus assay. Toxicol. Res. Appl. 2019, 3, 2397847319887904. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, P.; Bonnarme, V.; Piccirilli, A.; Ayrault, P.; Lemée, L.; Frapper, G.; Pourchez, J. Physical and chemical assessment of 1, 3 Propanediol as a potential substitute of propylene glycol in refill liquid for electronic cigarettes. Sci. Rep. 2018, 8, 10702. [Google Scholar] [CrossRef] [Green Version]
- Goenka, S. Effects of serotype and species dependency of bacterial lipopolysaccharides in human melanocytes from lightly and darkly-pigmented skin. BBA Adv. 2022, 2, 100042. [Google Scholar] [CrossRef]





| PG/VG | IC50 (% v/v) |
|---|---|
| 0/100 | 5.96 ± 0.97 |
| 20/80 | 6.18 ± 0.61 |
| 55/45 | 5.47 ± 1.34 |
| 80/20 | 3.76 ± 0.28 a |
| 100/0 | 4.70 ± 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S. Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes. Oral 2023, 3, 40-56. https://doi.org/10.3390/oral3010005
Goenka S. Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes. Oral. 2023; 3(1):40-56. https://doi.org/10.3390/oral3010005
Chicago/Turabian StyleGoenka, Shilpi. 2023. "Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes" Oral 3, no. 1: 40-56. https://doi.org/10.3390/oral3010005
APA StyleGoenka, S. (2023). Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes. Oral, 3(1), 40-56. https://doi.org/10.3390/oral3010005