Phosphorescence of Hydrogen-Capped Linear Polyyne Molecules C8H2, C10H2 and C12H2 in Solid Hexane Matrices at 20 K
Abstract
:1. Introduction
2. Materials and Methods
3. Results
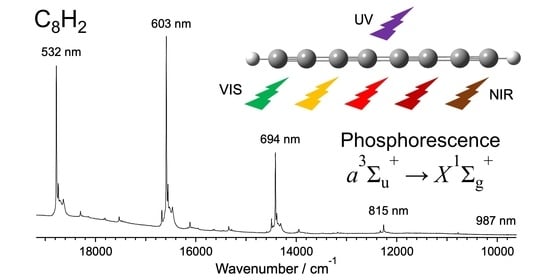
3.1. Phosphorescence Spectra and Excitation Spectra
3.2. Lifetimes
3.3. Vibrational Bands
3.4. Annealing of Matrix Samples
4. Discussion
4.1. Electronic Transition and Vibrational Excitation
4.2. Phosphorescence Lifetimes
4.3. Phosphorescence versus Fluorescence
4.4. Vibronic Bands in the Forbidden Transition
4.5. Relevance to Interstellar Molecules
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cataldo, F. (Ed.) Polyynes: Synthesis, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. (Eds.) Acetylene Chemistry: Chemistry, Biology, and Material Science; Wiley-VCH: Weinheim, Switzerland, 2004. [Google Scholar] [CrossRef]
- Broten, N.W.; Oka, T.; Avery, L.W.; MacLeod, J.M.; Kroto, H.W. The detection of HC9N in interstellar space. Astrophys. J. 1978, 223, L105. [Google Scholar] [CrossRef]
- Snell, R.L.; Schloerb, F.P.; Young, J.S.; Hjalmarson, A.; Friberg, P. Observations of HC3N, HC5N, and HC7N in molecular clouds. Astrophys. J. 1981, 244, 45–53. [Google Scholar] [CrossRef]
- Bell, M.B.; Avery, L.W.; MacLeod, J.M.; Matthews, H.E. The excitation temperature of HC9N in the circumstellar envelope of IRC+10216. Astrophys. J. 1992, 400, 551–555. [Google Scholar] [CrossRef]
- Truong-Bach, D.G. HC9N from the envelopes of IRC+10216 and CRL:2688. Astron. Astrophys. 1993, 277, 133–138. [Google Scholar]
- Loomis, R.A.; Shingledecker, C.N.; Langston, G.; McGuire, B.A.; Dollhopf, N.M.; Burkhardt, A.M.; Corby, J.; Booth, S.T.; Carroll, P.B.; Turner, B.; et al. Non-detection of HC11N towards TMC-1: Constraining the chemistry of large carbon-chain molecules. Mon. Not. R. Astron. Soc. 2016, 463, 4175–4183. [Google Scholar] [CrossRef] [Green Version]
- Heath, J.R.; Zhang, Q.; O’Brien, S.C.; Curl, R.F.; Kroto, H.W.; Smalley, R.E. The formation of long carbon chain molecules during laser vaporization of graphite. J. Am. Chem. Soc. 1987, 109, 359–363. [Google Scholar] [CrossRef]
- Eastmond, R.; Johnson, T.R.; Walton, D.R.M. Silylation as a protective method for terminal alkynes in oxidative couplings—A general synthesis of the parent polyynes H(C≡C)nH (n = 4–10, 12). Tetrahedron 1972, 28, 4601–4616. [Google Scholar] [CrossRef]
- Kloster-Jensen, E.; Haink, H.-J.; Christen, H. The electronic spectra of unsubstituted mono- to penta- acetylene in the gas phase and in solution in the range 1100 to 4000 Å. Helv. Chim. Acta 1974, 57, 1731–1744. [Google Scholar] [CrossRef]
- Shindo, F.; Bénilan, Y.; Chaquin, P.; Guillemin, J.-C.; Jolly, A.; Raulin, F. IR spectrum of C8H2: Integrated band intensities and some observational implications. J. Mol. Spectrosc. 2001, 210, 191–195. [Google Scholar] [CrossRef]
- Shindo, F.; Benilan, Y.; Guillemin, J.-C.; Chaquin, P.; Jolly, A.; Raulin, F. Ultraviolet and infrared spectrum of C6H2 revisited and vapor pressure curve in Titan’s atmosphere. Planet. Space Sci. 2003, 51, 9–17. [Google Scholar] [CrossRef]
- Coupeaud, A.; Kołos, R.; Couturier-Tamburelli, I.; Aycard, J.P.; Piétri, N. Photochemical synthesis of the cyanodiacetylene HC5N: A cryogenic matrix experiment. J. Phys. Chem. A 2006, 110, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Crépin, C.; Turowski, M.; Ceponkus, J.; Douin, S.; Boyé-Péronne, S.; Gronowski, M.; Kołos, R. UV-induced growth of cyanopolyyne chains in cryogenic solids. Phys. Chem. Chem. Phys. 2011, 13, 16780–16785. [Google Scholar] [CrossRef] [PubMed]
- Couturier-Tamburelli, I.; Piétri, N.; Crépin, C.; Turowski, M.; Guillemin, J.-C.; Kołos, R. Synthesis and spectroscopy of cyano-triacetylene (HC7N) in solid argon. J. Chem. Phys. 2014, 140, 044329. [Google Scholar] [CrossRef]
- Turowski, M.; Crépin, C.; Gronowski, M.; Guillemin, J.-C.; Coupeaud, A.; Couturier-Tamburelli, I.; Piétri, N.; Kołos, R. Electronic absorption and phosphorescence of cyanodiacetylene. J. Chem. Phys. 2010, 133, 074310. [Google Scholar] [CrossRef] [PubMed]
- Turowski, M.; Crépin, C.; Douin, S.; Gronowski, M.; Couturier-Tamburelli, I.; Piétri, N.; Wasiak, A.; Kołos, R. Low temperature Raman spectra of cyanobutadiyne (HC5N). Vib. Spectrosc. 2012, 62, 268–272. [Google Scholar] [CrossRef]
- Szczepaniak, U.; Crépin, C.; Gronowski, M.; Chevalier, M.; Guillemin, J.-C.; Turowski, M.; Custer, T.; Kołos, R. Cryogenic photochemical synthesis and electronic spectroscopy of cyanotetraacetylene. J. Phys. Chem. A 2017, 121, 7374–7384. [Google Scholar] [CrossRef]
- Szczepaniak, U.; Turowski, M.; Custer, T.; Gronowski, M.; Kerisit, N.; Trolez, Y.; Kołos, R. Infrared and Raman spectroscopy of methylcyanodiacetylene (CH3C5N). ChemPhysChem 2016, 17, 3047–3054. [Google Scholar] [CrossRef]
- Szczepaniak, U.; Kołos, R.; Gronowski, M.; Guillemin, J.-C.; Crépin, C. Low temperature synthesis and phosphorescence of methyltriacetylene. J. Phys. Chem. A 2018, 122, 89–99. [Google Scholar] [CrossRef]
- Turowski, M.; Szczepaniak, U.; Custer, T.; Gronowski, M.; Kołos, R. Electronic spectroscopy of methylcyanodiacetylene (CH3C5N). ChemPhysChem 2016, 17, 4068–4078. [Google Scholar] [CrossRef]
- Szczepaniak, U.; Ozaki, K.; Tanaka, K.; Ohnishi, Y.; Wada, Y.; Guillemin, J.-C.; Crépin, C.; Kołos, R.; Morisawa, Y.; Suzuki, H.; et al. Phosphorescence excitation mapping and vibrational spectroscopy of HC9N and HC11N cyanopolyynes in organic solvents. J. Mol. Struct. 2020, 1214, 128201. [Google Scholar] [CrossRef]
- Tsuji, M.; Tsuji, T.; Kuboyama, S.; Yoon, S.-H.; Korai, Y.; Tsujimoto, T.; Kubo, T.T.; Mori, A.; Mochida, I. Formation of hydrogen-capped polyynes by laser ablation of graphite particles suspended in solution. Chem. Phys. Lett. 2002, 355, 101–108. [Google Scholar] [CrossRef]
- Tabata, H.; Fujii, M.; Hayashi, M.; Doi, T.; Wakabayashi, T. Raman and surface-enhanced Raman scattering of a series of polyynes. Carbon 2006, 44, 3168–3176. [Google Scholar] [CrossRef]
- Inoue, K.; Matsutani, R.; Sanada, T.; Kojima, K. Preparation of long-chain polyynes of C24H2 and C26H2 by liquid-phase laser ablation in decalin. Carbon 2010, 48, 4209–4211. [Google Scholar] [CrossRef]
- Wada, Y.; Morisawa, Y.; Wakabayashi, T. Spectroscopic characterization of a series of polyyne-iodine molecular complexes H(C≡C)nH(I6) of n = 5–9. Chem. Phys. Lett. 2012, 541, 54–59. [Google Scholar] [CrossRef]
- Sato, Y.; Kodama, T.; Shiromaru, H.; Sanderson, J.H.; Fujino, T.; Wada, Y.; Wakabayashi, T.; Achiba, Y. Synthesis of polyyne molecules from hexane by irradiation of intense femtosecond laser pulses. Carbon 2010, 48, 1673–1676. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Tabata, H.; Doi, T.; Nagayama, H.; Okuda, K.; Umeda, R.; Hisaki, I.; Sonoda, M.; Tobe, Y.; Minematsu, T.; et al. Resonance Raman spectra of polyyne molecules C10H2 and C12H2 in solution. Chem. Phys. Lett. 2007, 433, 296–300. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Saikawa, M.; Wada, Y.; Minematsu, T. Isotope scrambling in the formation of cyanopolyynes by laser ablation of carbon particles in liquid acetonitrile. Carbon 2012, 50, 47–56. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Nagayama, H.; Daigoku, K.; Kiyooka, Y.; Hashimoto, K. Laser induced emission spectra of polyyne molecules C2nH2 (n = 5–8). Chem. Phys. Lett. 2007, 446, 65–70. [Google Scholar] [CrossRef]
- Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. Available online: https://gaussian.com/gaussian16/ (accessed on 21 February 2021).
- Ding, H.; Schmidt, T.W.; Pino, T.; Güthe, F.; Maier, J.P. Towards bulk behaviour of long hydrogenated carbon chains? Phys. Chem. Chem. Phys. 2003, 5, 4772–4775. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Wada, Y.; Iwahara, N.; Sato, T. Vibronic bands in the HOMO-LUMO excitation of linear polyyne molecules. J. Phys. Conf. Ser. 2013, 428, 012004. [Google Scholar] [CrossRef]
- Nakai, H. Discovery of chemical principles: Symmetry rules of degenerate excitations. J. Comp. Chem. Jpn. 2012, 11, 1–16. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Čermák, I.; Förderer, M.; Čermáková, I.; Kalhofer, S.; Stopka-Ebeler, H.; Monninger, G.; Krätschmer, W. Laser-induced emission spectroscopy of matrix-isolated carbon molecules: Experimental setup and new results on C3. J. Chem. Phys. 1998, 108, 10129–10142. [Google Scholar] [CrossRef]
- Orlandi, G.; Siebrand, W. Theory of vibronic intensity borrowing. Comparison of Herzberg-Teller and Born-Oppenheimer coupling. J. Chem. Phys. 1973, 58, 4513–4523. [Google Scholar] [CrossRef]
- Lin, S.H.; Eyring, H. Study of vibronic and Born-Oppenheimer couplings. Proc. Nat. Acad. Sci. USA 1974, 71, 3415–3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.H.; Eyring, H. Study of the Franck-Condon and Herzberg-Teller Approximations. Proc. Nat. Acad. Sci. USA 1974, 71, 3802–3804. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Molecule | Assignment 1 | Solid Matrix Hexane (20 K) | Solution 2 Hexane | Gas Phase 2 |
|---|---|---|---|---|
| C8H2 | 0–0 | 43,113 (231.9) | 43,960 (227.5) | 48,190 (207.5) |
| 1–0 | 45,243 (221.0) | 46,080 (217.0) | 50,280 (198.9) | |
| 2–0 | 48,190 (207.5) | 52,380 (190.9) | ||
| C10H2 | 0–0 | 38,728 (258.2) | 39,640 (252.3) | 43,690 (228.9) |
| 1–0 | 40,815 (245.0) | 41,670 (240.0) | 45,790 (218.4) | |
| 2–0 | 42,909 (233.1) | 43,630 (229.2) | 47,820 (209.1) | |
| 3–0 | 44,940 (222.5) | 45,600 (219.3) | ||
| C12H2 | 0–0 | 35,420 (282.3) | 36,260 (275.8) | 40,370 (247.7) |
| 1–0 | 37,511 (266.6) | 38,300 (261.1) | 42,340 (236.2) | |
| 2–0 | 39,469 (253.4) | 40,230 (248.6) | 44,250 (226.0) | |
| 3–0 | 41,457 (241.2) | 42,160 (237.2) | ||
| 4–0 | 43,292 (231.0) | 44,010 (227.2) |
| Molecule | Constant | Solid cm−1 | Solution cm−1 | Gas cm−1 |
|---|---|---|---|---|
| C8H2 | T * 1 | 42,048 | 42,896 | 47,145 |
| ωe’ | 2130 2 | 2130 | 2090 2 | |
| ωexe’ | – | 5 | – | |
| C10H2 | T * 1 | 37,663 | 38,626 | 42,614 |
| ωe’ | 2129 | 2044 | 2170 | |
| ωexe’ | 14 | 15 | 35 | |
| C12H2 | T * 1 | 34,361 | 35,237 | 39,362 |
| ωe’ | 2141 | 2072 | 2030 | |
| ωexe’ | 34 | 27 | 30 |
| Molecule | Observed Band cm−1 (nm) | Difference Frequency cm−1 | Vibrational Mode Assignment | ||
|---|---|---|---|---|---|
| C8H2 | 18,788 (532.3) | 0 | 0–0 band | ||
| 18,305 | 483 | σg’ | breathing b | ||
| and/or | πu’2 | cis-zigzag d | |||
| 17,819 | 969 | πu’4 | cis-zigzag d | ||
| 17,540 | 1248 | πg2 | trans-zigzag c | ||
| 16,597 (602.4) | 2190 | σg | alternating CC str. a | ||
| 16,126 | 471 | σg + σg’ | breathing b | ||
| and/or | σg + πu’2 | cis-zigzag a,d | |||
| 15,646 | 951 | σg + πu’4 | cis-zigzag a,d | ||
| 15,352 | 1245 | σg + πg2 | trans-zigzag a,c | ||
| 14,426 (693.2) | 2172 | σg2 | alternating CC str. a | ||
| 13,959 | 467 | σg2 + σg’ | breathing b | ||
| and/or | σg2 + πu’2 | cis-zigzag a,d | |||
| 13,183 | 1243 | σg2 + πg2 | trans-zigzag a,c | ||
| 12,268 (815.1) | 2157 | σg3 | alternating CC str. a | ||
| 10,133 (986.9) | 2136 | σg4 | alternating CC str. a | ||
| C10H2 | 16,525 (605.1) | 0 | 0–0 band | ||
| 16,145 | 380 | σg’ | breathing b | ||
| 16,042 | 483 | πg’2 | cis-zigzag d | ||
| 15,560 | 965 | πg’4 | cis-zigzag d | ||
| 15,293 | 1232 | πg2 | trans-zigzag c | ||
| 14,405 (694.2) | 2120 | σg | alternating CC str. a | ||
| 14,031 | 374 | σg + σg’ | breathing a,b | ||
| 13,930 | 475 | σg + πg’2 | cis-zigzag a,d | ||
| 13,456 | 949 | σg + πg’4 | cis-zigzag a,d | ||
| 13,172 | 1233 | σg + πg2 | trans-zigzag a,c | ||
| 12,300 (813.0) | 2105 | σg2 | alternating CC str. a | ||
| 11,902 | 398 | σg2 + σg’ | breathing a,b | ||
| 11,847 | 453 | σg2 + πg’2 | cis-zigzag a,d | ||
| 10,210 (979.4) | 2090 | σg3 | alternating CC str. a | ||
| C12H2 | 15,178 (658.8) | 0 | 0–0 band | ||
| 14,869 | 309 | σg’ | breathing b | ||
| 14,699 | 479 | πu’2 | cis-zigzag d | ||
| 14,205 | 973 | πu’4 | cis-zigzag d | ||
| 13,883 | 1295 | πg2 | trans-zigzag c | ||
| 13,088 (764.1) | 2090 | σg | alternating CC str. c | ||
| 12,765 | 323 | σg + σg’ | breathing a,b | ||
| 12,602 | 486 | σg + πu’2 | cis-zigzag a,d | ||
| 11,008 (908.4) | 2080 | σg2 | alternating CC str. a | ||
| 10,699 | 309 | σg2 + σg’ | breathing a,b |
| Molecule | T * 1 cm−1 | ωe” cm−1 | ωexe” cm−1 | Mode | Raman 2 cm−1 |
|---|---|---|---|---|---|
| C8H2 | 19,888 | 2205 | 8.1 | ν2σg | 2172 |
| C10H2 | 17,590 | 2135 | 7.5 | ν3σg | 2123 |
| C12H2 | 16,227 | 2100 | 5.0 | ν3σg | 2096 |
| Transition | Excitation 686.0 nm | Excitation 687.0 nm | Excitation 693.5 nm | Separation cm−1 | Solution 1 nm | Mode | Symmetry |
|---|---|---|---|---|---|---|---|
| 1Δu ← X1∑g+ | 395.3 | 392.8 | 397.8 | ||||
| 390.3 | 0 | ||||||
| 387.8 | 387.8 | 387.8 | 165 | 384 | πg2 | ||
| 381.8 | 571 | ||||||
| 379.3 | 173 | ||||||
| 365.3 | 363.8 | 367.3 | |||||
| 361.3 | 2057 | ||||||
| 359.3 | 359.3 | 359.3 | 154 | 357 | σg + πg2 | ||
| 353.8 | 587 | ||||||
| 351.8 | 161 | ||||||
| 339.8 | 339.8 | 340.8 | |||||
| 336.3 | 2058 | ||||||
| 334.8 | 334.8 | 334.8 | 133 | 333 | σg2 + πg2 | ||
| 330.3 | 540 | ||||||
| 312.8 | 313.8 | 314.8 | 2031 | 312 | σg3 + πg2 | ||
| 1Δu ← X1∑g+ | 270.2 | 271.2 | |||||
| 268.2 | 268.2 | ||||||
| 266.2 | 266.2 | ||||||
| 1∑u+ ← X1∑g+ | 261.7 | 261.7 | 258.8 | 0 | 0–0 | ||
| 255.3 | 257.3 | 245.3 | 2116 | σg | |||
| 250.8 | 251.3 | 233.4 | 2084 | σg2 | |||
| 242.3 | 242.8 | 222.9 | 2008 | σg3 | |||
| 233.9 | 239.9 | ||||||
| 230.9 | 231.4 | ||||||
| 222.9 | |||||||
| 220.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakabayashi, T.; Szczepaniak, U.; Tanaka, K.; Saito, S.; Fukumoto, K.; Ohnishi, R.; Ozaki, K.; Yamamoto, T.; Suzuki, H.; Guillemin, J.-C.; et al. Phosphorescence of Hydrogen-Capped Linear Polyyne Molecules C8H2, C10H2 and C12H2 in Solid Hexane Matrices at 20 K. Photochem 2022, 2, 181-201. https://doi.org/10.3390/photochem2010014
Wakabayashi T, Szczepaniak U, Tanaka K, Saito S, Fukumoto K, Ohnishi R, Ozaki K, Yamamoto T, Suzuki H, Guillemin J-C, et al. Phosphorescence of Hydrogen-Capped Linear Polyyne Molecules C8H2, C10H2 and C12H2 in Solid Hexane Matrices at 20 K. Photochem. 2022; 2(1):181-201. https://doi.org/10.3390/photochem2010014
Chicago/Turabian StyleWakabayashi, Tomonari, Urszula Szczepaniak, Kaito Tanaka, Satomi Saito, Keisuke Fukumoto, Riku Ohnishi, Kazunori Ozaki, Taro Yamamoto, Hal Suzuki, Jean-Claude Guillemin, and et al. 2022. "Phosphorescence of Hydrogen-Capped Linear Polyyne Molecules C8H2, C10H2 and C12H2 in Solid Hexane Matrices at 20 K" Photochem 2, no. 1: 181-201. https://doi.org/10.3390/photochem2010014
APA StyleWakabayashi, T., Szczepaniak, U., Tanaka, K., Saito, S., Fukumoto, K., Ohnishi, R., Ozaki, K., Yamamoto, T., Suzuki, H., Guillemin, J. -C., Shiromaru, H., Kodama, T., & Hatanaka, M. (2022). Phosphorescence of Hydrogen-Capped Linear Polyyne Molecules C8H2, C10H2 and C12H2 in Solid Hexane Matrices at 20 K. Photochem, 2(1), 181-201. https://doi.org/10.3390/photochem2010014