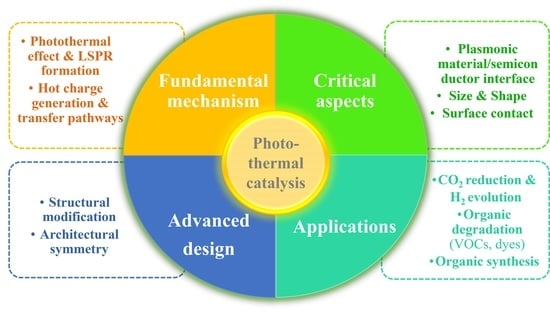
Plasmon-Induced Semiconductor-Based Photo-Thermal Catalysis: Fundamentals, Critical Aspects, Design, and Applications
Abstract
:1. Introduction
2. Fundamentals of LSPR Formation in Photo-Thermal Catalysis
3. Photo-Thermal Enhanced Catalysis
3.1. Indirect Transfer of the Hot Electron into the Adsorbate
3.2. Direct Transfer of Hot Electron into the Adsorbate
3.3. Indirect Transfer of Hot Electron into the Semiconductor
3.4. Direct Transfer of Hot Electron into the Semiconductor
4. Influential Aspects Contributing to Photo-Thermal Catalysts
4.1. Effect of Size and Shape
4.2. Designing the Hybrid-Structure System
4.2.1. Metal–Semiconductor Hybrid Systems
4.2.2. Metal–Porous Hybrid Systems
4.2.3. Core–Shell Hybrid Systems
5. Photo-Thermal Effect-Based Applications
5.1. Solar Fuel Generation
5.2. CO2 Reduction

5.3. H2 Evolution
5.4. Organic Compound Degradation and Production
6. Conclusions and Perspective
- Photo-thermal catalysis is far better than the other conventional technologies as it can perform efficiently under mild conditions, but the main challenge is to incorporate the light into photo-thermal systems; for that purpose, suitable photoreactors with higher solar energy harvesting and utilization efficiency are required. However, various reported studies indicated that the application of continuous flow systems is more feasible for large-scale implementation, instead of applying batch experimental systems. Thereby, more attention should be paid in this approach to develop highly efficient and configured reactors.
- Among the extensive amount of investigations available on photo-thermal catalysis, few have explained the dominant reaction pathway that governs the actual reaction mechanism. Fundamentally, more research in this area is required.
- Although several different plasmonic materials, other than the noble plasmonic metals, have been revealed in recent years, a lot still needs to be discovered. In recent years, chalcogenides and nitrides have been extensively applied in photo-thermal catalytic processes due to their efficient photo-thermal ability.
- For instance, the CO2 conversion process mainly yields CO and CH4; however, more attention should be paid towards other valuable alkanes and alkenes (olefins), such as propylene, formic acid, ethylene, etc. In addition, one of the most vigorous characteristics in photo-thermal catalysis is the selectivity control, so more information on this could be favorable for designing high-selectivity photo-thermal catalytic systems.
- The stability and recyclability of photocatalysts are also major challenges, as most stability assessment experiments are severely constrained to a few hours. Therefore, highly sustainable photocatalysts are urgently needed in order to meet these large-scale requirements.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Gao, W.; Li, S.; Williams, G.R.; Mahadi, A.H.; Ma, D. Solar-versus Thermal-Driven Catalysis for Energy Conversion. Joule 2019, 3, 920–937. [Google Scholar] [CrossRef] [Green Version]
- Jeong, G.H.; Sasikala, S.P.; Yun, T.; Lee, G.Y.; Lee, W.J.; Kim, S.O. Nanoscale Assembly of 2D Materials for Energy and Environmental Applications. Adv. Mater. 2020, 32, e1907006. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, H.S.; Qazi, R.; Kwon, Y.; Jeong, J.; Yeo, W. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2019, 32, e1901924. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Yang, H.Y. Recent Advances in Growth of Novel 2D Materials: Beyond Graphene and Transition Metal Dichalcogenides. Adv. Mater. 2018, 30, e1800865. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kong, Z.-C.; Liao, J.-F.; Dong, Y.-J.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Core@Shell CsPbBr3@Zeolitic Imidazolate Framework Nanocomposite for Efficient Photocatalytic CO2 Reduction. ACS Energy Lett. 2018, 3, 2656–2662. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-Induced Hot Carriers in Metallic Nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Richardson, H.H.; Hickman, Z.N.; Govorov, A.O.; Thomas, A.C.; Zhang, W.; Kordesch, M.E. Thermooptical Properties of Gold Nanoparticles Embedded in Ice: Characterization of Heat Generation and Melting. Nano Lett. 2006, 6, 783–788. [Google Scholar] [CrossRef]
- Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photon-Rev. 2019, 7, 170. [CrossRef]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal Membrane Distillation for Seawater Desalination. Adv. Mater. 2017, 29, 1603504. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Xia, M.; Duchesne, P.N.; Wang, L.; Mao, C.; Jelle, A.A.; Yan, T.; Li, P.; Lu, Z.-H.; Ozin, G.A. Enhanced CO2 Photocatalysis by Indium Oxide Hydroxide Supported on TiN@TiO2 Nanotubes. Nano Lett. 2021, 21, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Solar-driven photothermal nanostructured materials designs and prerequisites for evaporation and catalysis applications. Mater. Horiz. 2018, 5, 323–343. [Google Scholar] [CrossRef]
- Li, S.; Miao, P.; Zhang, Y.; Wu, J.; Zhang, B.; Du, Y.; Han, X.; Sun, J.; Xu, P. Recent Advances in Plasmonic Nanostructures for Enhanced Photocatalysis and Electrocatalysis. Adv. Mater. 2020, 33, e2000086. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.G.; Sandhel, A.; Wood, T.E.; Jelle, A.A.; Hoch, L.B.; Perovic, D.D.; Mims, C.A.; Ozin, G.A. Photomethanation of Gaseous CO2over Ru/Silicon Nanowire Catalysts with Visible and Near-Infrared Photons. Adv. Sci. 2014, 1, 1400001. [Google Scholar] [CrossRef]
- Jia, J.; O’Brien, P.G.; He, L.; Qiao, Q.; Fei, T.; Reyes, L.M.; Burrow, T.E.; Dong, Y.; Liao, K.; Varela, M.; et al. Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO2 over Nanostructured Pd@Nb2O5. Adv. Sci. 2016, 3, 1600189. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Lu, C.; Xu, Z. Photothermal application of SmCoO3/SBA-15 catalysts synthesized by impregnation method. Mater. Lett. 2018, 228, 199–202. [Google Scholar] [CrossRef]
- Vu, N.; Kaliaguine, S.; Do, T. Plasmonic Photocatalysts for Sunlight-Driven Reduction of CO2: Details, Developments, and Perspectives. ChemSusChem 2020, 13, 3967–3991. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Y.; Yu, L.; Huang, B. Energy transfer in plasmonic photocatalytic composites. Light Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; O’Donnell, S.; Sang, D.L.; Maggard, P.A.; Wang, G. Harnessing Plasmon-Induced Hot Carriers at the Interfaces With Ferroelectrics. Front. Chem. 2019, 7, 299. [Google Scholar] [CrossRef]
- Gellé, A.; Jin, T.; De La Garza, L.; Price, G.D.; Besteiro, L.V.; Moores, A. Applications of Plasmon-Enhanced Nanocatalysis to Organic Transformations. Chem. Rev. 2019, 120, 986–1041. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.B.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2012, 23, 1612–1619. [Google Scholar] [CrossRef]
- Qian, K.; Sweeny, B.C.; Johnston-Peck, A.C.; Niu, W.; Graham, J.O.; DuChene, J.S.; Qiu, J.; Wang, Y.-C.; Engelhard, M.H.; Su, D.; et al. Surface Plasmon-Driven Water Reduction: Gold Nanoparticle Size Matters. J. Am. Chem. Soc. 2019, 136, 9842–9845. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct Photocatalysis by Plasmonic Nanostructures. ACS Catal. 2014, 4, 116–128. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2014, 3, 467–472. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef]
- Hartland, G.V.; Besteiro, L.V.; Johns, P.; Govorov, A.O. What’s so Hot about Electrons in Metal Nanoparticles? ACS Energy Lett. 2017, 2, 1641–1653. [Google Scholar] [CrossRef] [Green Version]
- Christopher, P.; Moskovits, M. Hot Charge Carrier Transmission from Plasmonic Nanostructures. Annu. Rev. Phys. Chem. 2017, 68, 379–398. [Google Scholar] [CrossRef]
- Boerigter, C.; Campana, R.; Morabito, M.; Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 2016, 7, 10545. [Google Scholar] [CrossRef] [Green Version]
- Sarina, S.; Jaatinen, E.; Xiao, Q.; Huang, Y.M.; Christopher, P.; Zhao, J.C.; Zhu, H.Y. Photon Energy Threshold in Direct Photocatalysis with Metal Nanoparticles: Key Evidence from the Action Spectrum of the Reaction. J. Phys. Chem. Lett. 2017, 8, 2526–2534. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zhou, L.; Goodman, A.M.; Large, N.; Ayala-Orozco, C.; Zhang, Y.; Nordlander, P.; Halas, N.J. Hot-Electron-Induced Dissociation of H2on Gold Nanoparticles Supported on SiO2. J. Am. Chem. Soc. 2014, 136, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, A.; Zhang, J.; Linic, S. Tuning Selectivity in Propylene Epoxidation by Plasmon Mediated Photo-Switching of Cu Oxidation State. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot Electrons Do the Impossible: Plasmon-Induced Dissociation of H2 on Au. Nano Lett. 2012, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Abid, J.-P.; Fermin, D.; Girault, H.H. Ultrafast chemical interface scattering as an additional decay channel for nascent nonthermal electrons in small metal nanoparticles. J. Chem. Phys. 2004, 120, 9302–9315. [Google Scholar] [CrossRef] [PubMed]
- Boerigter, C.; Aslam, U.; Linic, S. Mechanism of Charge Transfer from Plasmonic Nanostructures to Chemically Attached Materials. ACS Nano 2016, 10, 6108–6115. [Google Scholar] [CrossRef]
- Foerster, B.; Joplin, A.; Kaefer, K.; Celiksoy, S.; Link, S.; Sönnichsen, C. Chemical Interface Damping Depends on Electrons Reaching the Surface. ACS Nano 2017, 11, 2886–2893. [Google Scholar] [CrossRef]
- Wang, M.; Ye, M.; Iocozzia, J.; Lin, C.; Lin, Z. Plasmon-Mediated Solar Energy Conversion via Photocatalysis in Noble Metal/Semiconductor Composites. Adv. Sci. 2016, 3, 1600024. [Google Scholar] [CrossRef] [Green Version]
- Baldoví, H.G.; Neaţu, S.; Khan, A.; Asiri, A.M.; Kosa, S.A.; Garcia, H. Understanding the Origin of the Photocatalytic CO2 Reduction by Au- and Cu-Loaded TiO2: A Microsecond Transient Absorption Spectroscopy Study. J. Phys. Chem. C 2015, 119, 6819–6827. [Google Scholar] [CrossRef]
- DuChene, J.S.; Sweeny, B.C.; Johnston-Peck, A.C.; Su, D.; Stach, E.A.; Wei, W.D. Prolonged Hot Electron Dynamics in Plasmonic-Metal/Semiconductor Heterostructures with Implications for Solar Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 7887–7891. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.N.K.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef]
- Marchuk, K.; Willets, K.A. Localized surface plasmons and hot electrons. Chem. Phys. 2014, 445, 95–104. [Google Scholar] [CrossRef]
- Schuck, P.J. Hot electrons go through the barrier. Nat. Nanotechnol. 2014, 8, 799–800. [Google Scholar] [CrossRef]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast Plasmon-Induced Electron Transfer from Gold Nanodots into TiO2 Nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- White, T.P.; Catchpole, K.R. Plasmon-enhanced internal photoemission for photovoltaics: Theoretical efficiency limits. Appl. Phys. Lett. 2012, 101, 073905. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Argondizzo, A.; Ren, J.; Liu, L.; Zhao, J.; Petek, H. Plasmonic coupling at a metal/semiconductor interface. Nat. Photonics 2017, 11, 806–812. [Google Scholar] [CrossRef]
- Yu, Y.; Ji, Z.; Zu, S.; Du, B.; Kang, Y.; Li, Z.; Zhou, Z.; Shi, K.; Fang, Z. Ultrafast Plasmonic Hot Electron Transfer in Au Nanoantenna/MoS2Heterostructures. Adv. Funct. Mater. 2016, 26, 6394–6401. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2020, 50, 2173–2210. [Google Scholar] [CrossRef]
- Ghoussoub, M.; Xia, M.; Duchesne, P.N.; Segal, D.; Ozin, G. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy Environ. Sci. 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Haque, S.S.; Zhang, M.; Xia, L. Localized surface plasmon resonance effects by naturally occurring Chinese yam particles. J. Appl. Phys. 2010, 108, 123502. [Google Scholar] [CrossRef]
- Forno, S.D.; Ranno, L.; Lischner, J. Material, Size, and Environment Dependence of Plasmon-Induced Hot Carriers in Metallic Nanoparticles. J. Phys. Chem. C 2018, 122, 8517–8527. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, W.; Moran, C.; Zeng, J.; Chen, J.; Wen, L.-P.; Xia, Y. Seed-Mediated Synthesis of Ag Nanocubes with Controllable Edge Lengths in the Range of 30−200 nm and Comparison of Their Optical Properties. J. Am. Chem. Soc. 2010, 132, 11372–11378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulvihill, M.J.; Ling, X.Y.; Henzie, J.; Yang, P. Anisotropic Etching of Silver Nanoparticles for Plasmonic Structures Capable of Single-Particle SERS. J. Am. Chem. Soc. 2010, 132, 268–274. [Google Scholar] [CrossRef]
- Zhang, H.; Govorov, A.O. Optical Generation of Hot Plasmonic Carriers in Metal Nanocrystals: The Effects of Shape and Field Enhancement. J. Phys. Chem. C 2014, 118, 7606–7614. [Google Scholar] [CrossRef]
- Besteiro, L.V.; Govorov, A.O. Amplified Generation of Hot Electrons and Quantum Surface Effects in Nanoparticle Dimers with Plasmonic Hot Spots. J. Phys. Chem. C 2016, 120, 19329–19339. [Google Scholar] [CrossRef]
- Lim, J.; Hippalgaonkar, K.; Andrews, S.C.; Majumdar, A.; Yang, P. Quantifying Surface Roughness Effects on Phonon Transport in Silicon Nanowires. Nano Lett. 2012, 12, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jiao, S.; Kang, Y.; Pang, G.; Feng, S. Photothermal Conversion of W18 O49 with a Tunable Oxidation State. ChemistryOpen 2017, 6, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Y.; Yan, T.; Hu, Z.; Jelle, A.A.; Meira, D.M.; Duchesne, P.N.; Loh, J.Y.Y.; Qiu, C.; Storey, E.E.; et al. Black indium oxide a photothermal CO2 hydrogenation catalyst. Nat. Commun. 2020, 11, 2432. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, X.; Zhu, H. Zeolite-Supported Gold Nanoparticles for Selective Photooxidation of Aromatic Alcohols under Visible-Light Irradiation. Chem.–A Eur. J. 2012, 18, 8048–8056. [Google Scholar] [CrossRef]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Q.; Marcus, K.; Hao, Y.; Deng, J.; Bi, K.; Yang, Y. Photocatalytic glycerol oxidation on AuxCu–CuS@TiO2 plasmonic heterostructures. J. Mater. Chem. A 2018, 6, 22005–22012. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Lv, Z.; Long, R.; Zhang, C.; Lin, Y.; Wei, K.; Wang, C.; Chen, L.; Li, Z.-Y.; et al. Unraveling Surface Plasmon Decay in Core–Shell Nanostructures toward Broadband Light-Driven Catalytic Organic Synthesis. J. Am. Chem. Soc. 2016, 138, 6822–6828. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.-D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.-H. Plasmonic Harvesting of Light Energy for Suzuki Coupling Reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef]
- Manthiram, K.; Alivisatos, A.P. Tunable Localized Surface Plasmon Resonances in Tungsten Oxide Nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Jiao, Y.; Zhou, X.; Wu, A.; Buhe, B.; Fu, H. Strongly coupled Ag/TiO2 heterojunctions for effective and stable photothermal catalytic reduction of 4-nitrophenol. Nano Res. 2017, 11, 126–141. [Google Scholar] [CrossRef]
- Lai, B.-H.; Lin, Y.-R.; Chen, D.-H. Fabrication of LaB6@SiO2/Au composite nanoparticles as a catalyst with near infrared photothermally enhanced activity. Chem. Eng. J. 2013, 223, 418–424. [Google Scholar] [CrossRef]
- Wang, C.; Ranasingha, O.; Natesakhawat, S.; Ohodnicki, P.R.; Andio, M.; Lewis, J.P.; Matranga, C. Visible light plasmonic heating of Au–ZnO for the catalytic reduction of CO2. Nanoscale 2013, 5, 6968–6974. [Google Scholar] [CrossRef]
- Zou, J.; Si, Z.; Cao, Y.; Ran, R.; Wu, X.; Weng, D. Localized Surface Plasmon Resonance Assisted Photothermal Catalysis of CO and Toluene Oxidation over Pd–CeO2 Catalyst under Visible Light Irradiation. J. Phys. Chem. C 2016, 120, 29116–29125. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Xu, H.; Meng, X.; Wang, T.; Wang, D.; Ye, J. Targeting Activation of CO2 and H2over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2016, 7, 1601657. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Wang, J.; Liu, H.; Dao, T.D.; Li, M.; Liu, G.; Meng, X.; Chang, K.; Shi, L.; et al. Surface-Plasmon-Enhanced Photodriven CO2 Reduction Catalyzed by Metal–Organic-Framework-Derived Iron Nanoparticles Encapsulated by Ultrathin Carbon Layers. Adv. Mater. 2016, 28, 3703–3710. [Google Scholar] [CrossRef]
- Robatjazi, H.; Zhao, H.; Swearer, D.F.; Hogan, N.J.; Zhou, L.; Alabastri, A.; McClain, M.J.; Nordlander, P.; Halas, N.J. Plasmon-induced selective carbon dioxide conversion on earth-abundant aluminum-cuprous oxide antenna-reactor nanoparticles. Nat. Commun. 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Hu, Y.; Jin, G.; Jiang, B.; Huang, Y. Photothermal-conversion-enhanced photocatalytic activity of flower-like CuS superparticles under solar light irradiation. Sol. Energy 2018, 170, 586–593. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Yu, S.; Jiang, H.-L. Pd Nanocubes@ZIF-8: Integration of Plasmon-Driven Photothermal Conversion with a Metal-Organic Framework for Efficient and Selective Catalysis. Angew. Chem. Int. Ed. 2016, 55, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Zeng, M.; Mao, M.; Lan, L.; Liu, H.; Chen, J.; Zhao, X. UV–vis-infrared light-driven photothermocatalytic abatement of CO on Cu doped ramsdellite MnO2 nanosheets enhanced by a photoactivation effect. Appl. Catal. B Environ. 2017, 224, 751–760. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Jiang, D.; Zhang, L.; Li, X.; Wang, Z. Ultrathin mesoporous Co3O4 nanosheets with excellent photo-/thermo-catalytic activity. J. Mater. Chem. A 2015, 4, 105–112. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Li, H.; Li, P.; Zou, Z. Au@TiO2yolk–shell hollow spheres for plasmon-induced photocatalytic reduction of CO2 to solar fuel via a local electromagnetic field. Nanoscale 2015, 7, 14232–14236. [Google Scholar] [CrossRef]
- Feng, S.; Wang, M.; Zhou, Y.; Li, P.; Tu, W.; Zou, Z. Double-shelled plasmonic Ag-TiO2 hollow spheres toward visible light-active photocatalytic conversion of CO2 into solar fuel. APL Mater. 2015, 3, 104416. [Google Scholar] [CrossRef] [Green Version]
- Do, J.Y.; Chava, R.K.; Mandari, K.K.; Park, N.-K.; Ryu, H.-J.; Seo, M.W.; Lee, D.; Senthil, T.; Kang, M. Selective methane production from visible-light-driven photocatalytic carbon dioxide reduction using the surface plasmon resonance effect of superfine silver nanoparticles anchored on lithium titanium dioxide nanocubes (Ag@LixTiO2). Appl. Catal. B Environ. 2018, 237, 895–910. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Yu, J.; Ho, W. Enhanced Photocatalytic Activity and Selectivity for CO 2 Reduction over a TiO2 Nanofibre Mat Using Ag and MgO as Bi-Cocatalyst. ChemCatChem 2019, 11, 465–472. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold–Copper Nanoalloys Supported on TiO2 as Photocatalysts for CO2 Reduction by Water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, B.; Meng, L.; He, S.; Yuan, R.; Hou, Y.; Ding, Z.; Lin, H.; Zhang, Z.; Wang, X.; et al. Plasmonic control of solar-driven CO2 conversion at the metal/ZnO interfaces. Appl. Catal. B Environ. 2019, 256, 117823. [Google Scholar] [CrossRef]
- Xin, Z.; Lu, L.; Wang, B.; Wang, X.; Zhu, K.; Xu, Z.; Yu, Z.; Yan, S.; Zou, Z. Lewis acid activated CO2 reduction over a Ni modified Ni–Ge hydroxide driven by visible-infrared light. Dalton Trans. 2018, 48, 1672–1679. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Z.; Han, S.; Zhang, H.; Xu, H.; Xu, C.; Ding, Z.; Yuan, R.; Chen, J.; Long, J. Plasmonic Electrons-Driven Solar-to-Hydrocarbon Conversion over Au NR@ZnO Core-Shell Nanostructures. ChemCatChem 2020, 12, 2989–2994. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Takeda, H.; Ishitani, O. Photocatalytic reduction of CO2 using metal complexes. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 106–137. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Wang, Z.U.; Wang, H.; Lu, J.; Yu, S.-H.; Jiang, H.-L. Singlet Oxygen-Engaged Selective Photo-Oxidation over Pt Nanocrystals/Porphyrinic MOF: The Roles of Photothermal Effect and Pt Electronic State. J. Am. Chem. Soc. 2017, 139, 2035–2044. [Google Scholar] [CrossRef]
- Kumar, D.; Park, C.H.; Kim, C.S. Robust Multimetallic Plasmonic Core–Satellite Nanodendrites: Highly Effective Visible-Light-Induced Colloidal CO2 Photoconversion System. ACS Sustain. Chem. Eng. 2018, 6, 8604–8614. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal Conversion of CO2 into CH4 with H2 over Group VIII Nanocatalysts: An Alternative Approach for Solar Fuel Production. Angew. Chem. 2014, 126, 11662–11666. [Google Scholar] [CrossRef]
- Chen, G.; Gao, R.; Zhao, Y.; Li, Z.; Waterhouse, G.I.N.; Shi, R.; Zhao, J.; Zhang, M.; Shang, L.; Sheng, G.; et al. Alumina-Supported CoFe Alloy Catalysts Derived from Layered-Double-Hydroxide Nanosheets for Efficient Photothermal CO2 Hydrogenation to Hydrocarbons. Adv. Mater. 2017, 30, 1704663. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Cheng, Y.; Liu, Z.; Guo, Q.; Ha, M.N.; Zhao, Z. Hydrogen-treated mesoporous WO3 as a reducing agent of CO2 to fuels (CH4 and CH3OH) with enhanced photothermal catalytic performance. J. Mater. Chem. A 2016, 4, 5314–5322. [Google Scholar] [CrossRef]
- Ha, M.N.; Lu, G.; Liu, Z.; Wang, L.; Zhao, Z. 3DOM-LaSrCoFeO6−δ as a highly active catalyst for the thermal and photothermal reduction of CO2 with H2O to CH4. J. Mater. Chem. A 2016, 4, 13155–13165. [Google Scholar] [CrossRef]
- Ma, R.; Sun, J.; Li, D.H.; Wei, J.J. Review of synergistic photo-thermo-catalysis: Mechanisms, materials and applications. Int. J. Hydrog. Energy 2020, 45, 30288–30324. [Google Scholar] [CrossRef]
- Peh, C.K.N.; Gao, M.; Ho, G.W. Harvesting broadband absorption of the solar spectrum for enhanced photocatalytic H2 generation. J. Mater. Chem. A 2015, 3, 19360–19367. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ye, L.; Ma, Z.; Han, C.; Wang, L.; Jia, Z.; Su, F.; Xie, H. Photothermal effect of infrared light to enhance solar catalytic hydrogen generation. Catal. Commun. 2017, 102, 13–16. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Agostini, G.; Marini, C.; Kubacka, A.; Fernández-García, M. Hydrogen thermo-photo production using Ru/TiO2: Heat and light synergistic effects. Appl. Catal. B Environ. 2019, 256, 117790. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, C.; Guo, Y.; Gao, J.; Yu, Y.; Zhang, B. Enhancing Electrocatalytic Water Splitting Activities via Photothermal Effect over Bifunctional Nickel/Reduced Graphene Oxide Nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 3710–3714. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Peng, T.; Xu, J.; Zhao, X. Photothermocatalytic Synergetic Effect Leads to High Efficient Detoxification of Benzene on TiO2 and Pt/TiO2 Nanocomposite. ChemCatChem 2010, 2, 1082–1087. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Shi, W.; Zhao, L.; Zhao, X.; Fang, P.; Zheng, F.; Wang, S. Novel effect of significant enhancement of gas-phase photocatalytic efficiency for nano ZnO. Chem. Eng. J. 2012, 213, 218–224. [Google Scholar] [CrossRef]
- Kim, J.-H.; Twaddle, K.M.; Hu, J.; Byun, H. Sunlight-Induced Synthesis of Various Gold Nanoparticles and Their Heterogeneous Catalytic Properties on a Paper-Based Substrate. ACS Appl. Mater. Interfaces 2014, 6, 11514–11522. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Valence state heterojunction Mn3O4/MnCO3: Photo and thermal synergistic catalyst. Appl. Catal. B Environ. 2015, 180, 6–12. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Lin, Y.; Mao, Y.; Ouyang, G.; Liu, H.; Zhang, S.; Tong, Y. Cerium-based hybrid nanorods for synergetic photo-thermocatalytic degradation of organic pollutants. J. Mater. Chem. A 2018, 6, 24740–24747. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Y.; Chai, Z.; Zeng, M.; Li, Q.; Wang, Y.; Xu, D. Photothermal-enhanced catalysis in core–shell plasmonic hierarchical Cu7S4microsphere@zeolitic imidazole framework-8. Chem. Sci. 2016, 7, 6887–6893. [Google Scholar] [CrossRef] [PubMed]








| Photocatalysts | Plasmonic NPs | Light Source | Absorption Wavelength (nm) | Temperature (°C) | Application | Ref. |
|---|---|---|---|---|---|---|
| Au-Pd nanostructures | Au nanorods | 1.68 W Laser, 809 nm | ca. 809 | 62 | Suzuki coupling reactions | [62] |
| Pd/WO3−x | WO3−x | Xe lamp | 400–1100 | 60 | Suzuki coupling reactions | [63] |
| Ag-TiO2 | Ag | 150 W Xe lamp | 200–1200 | - | 4-NP reduction | [64] |
| LaB6@SiO2/Au | LaB6 | CW 808 nm diode laser, 808 nm, 2.7 W m−2 | 400–1200 | 40.5 | 4-NP reduction | [65] |
| Aue-ZnO | Au | Laser, 532 nm, 8 × 105 Wm−2 | ca. 538 | 600 | CO2 reduction | [66] |
| Group VIII metals/Al2O3 | Group VIII metal | 300 W Xe lamp | 300–2500 | 300–400 | CO2 reduction | [67] |
| Ru@FL-LDHs | Ru | 300 W Xe lamp | 250–1500 | 350 | CO2 reduction | [68] |
| Fe@C | Fe | 300 W Xe lamp | 300–2500 | 481 | CO2 reduction | [69] |
| Al@Cu2O | Al | 10 Wcm−2, VIS-light | 300–1000 | 175 | CO2 reduction | [70] |
| Pd@Nb2O5 | Pd | 300 W Xe lamp, 18 kW m−2 | 250–2400 | 200 | CO2 reduction | [15] |
| Ru/SiNW | SiNW | Xe lamp | 300–2500 | 125 | CO2 reduction | [14] |
| Cu7S4@ZIF-8 | Cu7S4 | Xe lamp, 700 mW cm−2 | 250–2500 | 122 | Cyclocondensation reaction | [71] |
| Flower-like CuS | CuS | Xe lamp, 10 kW m−2 | 300–900 | 65 | Methylene blue degradation | [72] |
| Pd NCs@ZIF-8 | Pd Ncs | Xe lamp, 100 mW cm−2 | 220–700 | 34 | Hydrogenation reactions | [73] |
| Cu-RM-C | MnO2 | Xe lamp, 96.3 mW m−2 | 200–2400 | 120 | VOC oxidation | [74] |
| SS-Co3O4 | Co3O4 | Xe lamp, 500 mWcm−2 | 200–1500 | 175 | VOC oxidation | [75] |
| Au@TiO2 yolk shell | Au | 300 W Xe lamp | - | - | Methane production | [76] |
| Ag-TiO2 hollow-sphere | Ag | 300 W Xe lamp (λ > 420 nm) | - | - | Methane production | [77] |
| Ag@LixTiO2 nanocubes | Ag | Xe lamp 100 mW/cm2 (λ = 420 nm) | - | - | Methane production | [78] |
| Ag-MgO-TiO2 nanofibrous | Ag | 300 W Xe lamp | - | - | Methane production | [79] |
| Au-Cu alloys/TiO2 | Au-Cu | 1000 W Xe lamp AM 1.5 filter | - | - | Methane production | [80] |
| Pd-ZnO nanosheets | Pd | 300 W Xe lamp | - | - | Methane production | [81] |
| Ni-modified Ni-Gehydroxide | Ni | 300 W Xe lamp | - | - | CO generation | [82] |
| Au NR@ZnO | Au | 300 W Xe lamp | - | - | CO generation | [83] |
| Ag-ZnO nanosheets | Ag | 300 W Xe lamp | - | - | CO generation | [81] |
| Al@Cu2O antenna heterostructures | Al | Supercontinuum fiber laser (400 < λ < 850 nm) | - | - | CO generation | [70] |
| P25-rGO | rGO | Xe lamp | 250–800 | 36 | MB reduction | [84] |
| Pt/PCN-224(Zn) | PCN-224(Zn) | Xe lamp | 300–800 | 36 | Benzyl alcohol oxidation | [85] |
| Pt@TiO2-Au nanodendrites | Au | Xe lamp, 5.71 Wcm−2 | - | - | Methyl alcohol production | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sial, A.; Dar, A.A.; Li, Y.; Wang, C. Plasmon-Induced Semiconductor-Based Photo-Thermal Catalysis: Fundamentals, Critical Aspects, Design, and Applications. Photochem 2022, 2, 810-830. https://doi.org/10.3390/photochem2040052
Sial A, Dar AA, Li Y, Wang C. Plasmon-Induced Semiconductor-Based Photo-Thermal Catalysis: Fundamentals, Critical Aspects, Design, and Applications. Photochem. 2022; 2(4):810-830. https://doi.org/10.3390/photochem2040052
Chicago/Turabian StyleSial, Atif, Afzal Ahmed Dar, Yifan Li, and Chuanyi Wang. 2022. "Plasmon-Induced Semiconductor-Based Photo-Thermal Catalysis: Fundamentals, Critical Aspects, Design, and Applications" Photochem 2, no. 4: 810-830. https://doi.org/10.3390/photochem2040052
APA StyleSial, A., Dar, A. A., Li, Y., & Wang, C. (2022). Plasmon-Induced Semiconductor-Based Photo-Thermal Catalysis: Fundamentals, Critical Aspects, Design, and Applications. Photochem, 2(4), 810-830. https://doi.org/10.3390/photochem2040052