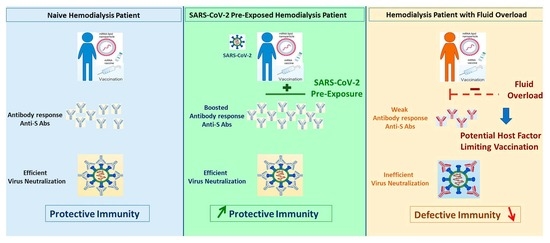
SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Assessment of Biological Parameters
2.3. Vaccine Protocol
2.4. Evaluation of the Antibody Response
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Patients and Control Group Characteristics
3.2. Biological Parameters Assessment
3.3. Tolerability of the SARS-CoV-2 mRNA Vaccine
3.4. Anti-S Antibody Titer Development after Vaccination in Naïve HD Patients and Control Subjects
3.5. Anti-S Antibody Titer Development in HD Patients Previously Exposed to SARS-CoV-2
3.6. Relative Fluid Overload (FO) and Anti-S Antibodies in Naïve Dialysis Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Perry Wilson, F.; et al. Characteristics and Outcomes of Individuals with Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203. [Google Scholar] [CrossRef] [PubMed]
- De Meester, J.; de Bacquer, D.; Naesens, M.; Meijers, B.; Couttenye, M.M.; de Vriese, A.S.; NBVN Kidney Registry Group. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: A regionwide registry study. J. Am. Soc. Nephrol. 2021, 32, 385–396. [Google Scholar] [CrossRef]
- Hsu, C.M.; Weiner, D.E.; Aweh, G.; Miskulin, D.C.; Manley, H.J.; Stewart, C.; Ladik, V.; Hosford, J.; Lacson, E.C.; Johnson, D.S.; et al. COVID-19 Infection Among US Dialysis Patients: Risk Factors and Outcomes from a National Dialysis Provider. Am. J. Kidney Dis. 2021, 77, 748–756. [Google Scholar] [CrossRef]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Zitt, E.; Davidovic, T.; Schimpf, J. The Safety and Immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 Vaccine in Hemodialysis Patients. Front. Immunol. 2021, 12, 704773. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Bhat, P.; Del Pilar Fernandez, M.; Bhat, J.G.; Coritsidis, G.N. COVID-19 Infection in ESKD: Findings from a Prospective Disease Surveillance Program at Dialysis Facilities in New York City and Long Island. J. Am. Soc. Nephrol. 2020, 31, 2517–2521. [Google Scholar] [CrossRef]
- Nongnuch, A.; Ngampongpan, W.; Srichatrapimuk, S.; Wongsa, A.; Thongpraphai, S.; Boonarkart, C.; Sanmeema, N.; Chittaganpitch, M.; Auewarakul, P.; Tassaneetrithep, B.; et al. Immune response to influenza vaccination in ESRD patients undergoing hemodialysis vs. hemodiafiltration. PLoS ONE 2020, 15, e0227719. [Google Scholar]
- Clarke, C.; Prendecki, M.; Dhutia, A.; Ali, M.A.; Sajjad, H.; Shivakumar, O.; Lightstone, L.; Kelleher, P.; Pickering, M.C.; Thomas, D.; et al. High Prevalence of Asymptomatic COVID-19 Infection in Hemodialysis Patients Detected Using Serologic Screening. J. Am. Soc. Nephrol. 2020, 31, 1969–1975. [Google Scholar] [CrossRef]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddiya, I. Current Knowledge of Vaccinations in Chronic Kidney Disease Patients. Int. J. Nephrol. Renovasc. Dis. 2020, 13, 179–185. [Google Scholar] [CrossRef] [PubMed]
- ERA-EDTA Council, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Basic science and dialysis: Disturbances of acquired immunity in hemodialysis patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; del Giudice, G.; Didierlaurent, A.M.; da Silva, F.T. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Broeders, N.E.; Hombrouck, A.; Lemy, A.; Wissing, K.M.; Racapé, J.; Gastaldello, K.; Massart, A.; van Gucht, S.; Weichselbaum, L.; de Mul, A.; et al. Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: A cohort study. Clin. J. Am. Soc. Nephrol. 2011, 6, 2573–2578. [Google Scholar] [CrossRef] [Green Version]
- Batty, C.; Bachelder, E.M.; Ainslie, K.M. Historitical Perspective of Clinical Nano and Microparticle Formulation for Delivery of Therapeutics. Forum Ser. Bottlenecks Breakthr. Mol. Med. 2021, 27, 516–519. [Google Scholar]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261. [Google Scholar] [CrossRef] [Green Version]
- Pepini, T.; Pulichino, A.; Carsillo, T.; Carlson, A.L.; Sari-Sarraf, F.; Ramsauer, K.; Debasitis, J.C.; Maruggi, G.; Otten, G.R.; Geall, A.J.; et al. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 2017, 198, 4012–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guermonprez, P.; Saveanu, L.; Kleijmeer, M.; Davoust, J.; Endert Van, P.; Amigorena, S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 2003, 425, 397–402. [Google Scholar] [CrossRef] [PubMed]
- El-Barbry, H.; Capitao, M.; Barrin, S.; Amziani, S.; Paul, P.P.; Borreill, S.; Guilbert, T.; Donnadieu, E.; Niedergang, F.; Ouaaz, F. Extracellular Release of Antigen by Dendritic Cell Regurgitation Promotes B Cell Activation through NF-κB/cRel. J. Immunol. 2020, 205, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Inaguma, D.; Koshi-Ito, E.; Ogata, S.; Kitagawa, A.; Takahashi, K.; Koide, S.; Hayashi, H.; Hasegawa, M.; Yuzawa, Y.; et al. Extreme hyperuricemia is a risk factor for infection-related deaths in incident dialysis patients: A multicenter prospective cohort study. Ren. Fail. 2020, 42, 646–655. [Google Scholar] [CrossRef]
- Durand, P.Y.; Nicco, C.; Serteyn, D.; Attaf, D.; Edeas, M. Microbiota Quality and Mitochondrial Activity Link with Occurrence of Muscle Cramps in Hemodialysis Patients using Citrate Dialysate: A Pilot Study. Blood Purif. 2018, 46, 301–308. [Google Scholar] [CrossRef]
- Alvarenga, L.A.; Andrade, B.D.; Moreira, M.A.; Nascimento, R.P.; Macedo, I.D.; Aguiar, A.S. Nutritional profile of hemodialysis patients concerning treatment time. J. Bras. Nefrol. 2017, 39, 283–286. [Google Scholar] [CrossRef]
- Ulrich, C.; Wilke, A.; Schleicher, N.; Girndt, M.; Fiedler, R. Hypervolemia-induced Disturbances Do Not involve IL-1 ss but IL-6 and IL-10 activation in hemodialysis patients. Toxins 2020, 12, 159. [Google Scholar] [CrossRef] [Green Version]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef]
- Hsu, H.-W.; Lang, C.-L.; Wang, M.-H.; Chiang, C.-K.; Lu, K.-C. A review of Chronic Kidney Disease and the Immune System: A special Form of Immunosenescence. J. Gerontol. Geriat. Res. 2014, 3, 1000144. [Google Scholar]
- Cook, P.C.; MacDonald, A.S. Dendritic cells in Lung immunopathology. Semin. Immunopathol. 2016, 38, 449–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-C.; Chiu, Y.-W.; Tsai, J.-C.; Kuo, H.-T.; Hung, C.-C.; Hwang, S.-J.; Chen, T.-H.; Kuo, M.-C.; Chen, H.-C. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, S.M.; Fildes, J.E.; Puchałka, C.M.; Basith, M.; Yonan, N.; Williams, S.G. BNP directly regulates the innate immune system of cardiac transplant recipients in vitro. Transpl. Immunol. 2009, 20, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Azzam, Z.S.; Kinaneh, S.; Fadel Bahouth, F.; Ismael-Badarneh, R.; Khoury, E.; Abassi, Z. Involvement of Cytokines in the Pathogenesis of Salt and Water Imbalance in Congestive Heart Failure. Front. Immunol. 2017, 8, 716. [Google Scholar] [CrossRef] [Green Version]
- Peesapati, V.S.R.; Sadik, M.; Verma, S.; Attallah, M.A.; Khan, S. Panoramic Dominance of the Immune System in Cardiorenal Syndrome Type, I. Cureus 2020, 12, e9869. [Google Scholar] [CrossRef]
- Cohen-Hagai, K.; Rozenberg, I.; Korzets, Z.; Zitman-Gal, T.; Einbinder, Y.; Benchetrit, S. Upper Respiratory Tract Infection among Dialysis Patients. Isr. Med. Assoc. J. 2016, 18, 557–560. [Google Scholar]
- Su, G.; Iwagami, M.; Qin, X.; McDonald, H.; Liu, X.; Carrero, J.J.; Lundborg, C.S.; Nitsch, D. Kidney disease and mortality in patients with respiratory tract infections: A systematic review and meta-analysis. Clin. Kidney J. 2021, 14, 602–611. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Dialysis Group | Control Group |
|---|---|---|
| Demographic (median, 25th–75th percentile) | ||
| Age (year) | 60 (49.5–69) | 45 (35–55) |
| Sex count (Male/Female) | 49/38 | 9/15 |
| Dry Body Weight (kg) | 74 (61.8–83) | 66 (56–71) |
| Height (cm) | 166 (163–170.5) | 167 (164–168) |
| Body mass index (BMI: kg/m2) | 25.4 (22.7–31.6) | 24 (23–26) |
| COVID-19 History (n, %) | ||
| Symptomatic | 7 (8.0%) | 3 (13.0%) |
| Asymptomatic | 12 (13.8%) | 1 (4.3%) |
| None | 68 (78.2%) | 19 (82.6%) |
| Comorbidities (n, %) | ||
| Charlson Index | 4.13 ± 1.18 | |
| Diabetes | 16 (18.39%) | 0 (0.00%) |
| Hypertension | 85 (97.70%) | 2 (8.69%) |
| Medication (n, %) | ||
| Antihypertensive | 80 (91.95%) | – |
| Cardiovascular medication | 38 (43.68%) | – |
| Phosphate binders | 58 (66.67%) | – |
| Carbonat of calcium | 50 (57.47%) | – |
| Cholecalciferol | 85 (97.70%) | – |
| Iron | 87 (100.0%) | – |
| Vitamin K antagonist | 5 (5.75%) | – |
| Nephropathy (n, %) | ||
| Glomerular | 1 (1.15%) | – |
| Vascular | 62 (71.26%) | – |
| Diabetic | 15 (17.24%) | – |
| Autosomal Polycystic Kidney Disease | 3 (3.45%) | – |
| HIV | 3 (3.45%) | – |
| Other | 11 (12.64%) | – |
| Renal Replacement Therapy | ||
| Dialysis vintage (Months) | 45.19 ± 37.39 | – |
| Past History of renal transplantation (n, %) | 8 (9.19%) | – |
| Immunossupressive therapy past/active (n, %) | 3 (3.45%) | – |
| Hemodialysis Parameters | |
|---|---|
| Treatment Schedule and Modality | |
| Weekly treatment time (min/week) | 743.45 ± 39.93 |
| High flux HD mode (%) | 87 (100%) |
| Conventional High Flux HD (n, %) | 45 (51.72%) |
| HDF (n, %) | 37 (42.53%) |
| Short Daily Home HD (n, %) | 5 (5.75%) |
| Anticoagulation (IU/session) | 2686.21 ± 1272.97 |
| Vascular access | |
| Native Arteriovenous Fistula (n, %) | 81 (93.10%) |
| Central Venous Catheter (n, %) | 6 (6.90%) |
| Dialysis Dose Delivered | |
| Kt/V (std, 87 pts) | 1.628 ± 0.35 |
| Kt/V (Weekly, 5 pts) | 2.25 ± 0.21 |
| Convective volume (L/session) | 24.80 ± 3.05 |
| Residual Urinary Output (n > 300 mL/day) | 50 (57.47%) |
| Blood Pressure (BP) * (mmHg) | |
| Pre-dialysis Systolic BP | 141.71 ± 22.66 |
| Pre-dialysis Diastolic BP | 68.43 ± 15.26 |
| Post-dialysis Systolic BP | 134.74 ± 20.5 |
| Post-dialysis Diastolic BP | 63.92 ± 13.83 |
| Fluid status (bioimpedence measurement) | |
| Fluid Overload (L) | 1.31 ± 2.09 |
| Dry weight (kg) | 75.75 ± 18.02 |
| Ultrafiltation (mL/session) | 2090.3 ± 928.77 |
| Ultrafiltation (mL/h/kg) | 7.22 ± 3.22 |
| Nutrition, Anemia & Inflammation Markers | |
| nPCR (g/kg/24 h) | 1.05 ± 0.22 |
| Hemoglobin level (g/L) | 11.26 ± 1.28 |
| Ferritin (µg/L) | 470.7 ± 228.29 |
| Transferin Saturation coefficient (%) | 26.84 ± 12.49 |
| Albumin (g/L) | 39.88 ± 3.33 |
| C-reactive protein (mg/L) | 7.36 ± 9.73 |
| Dialysis Patients | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Shot | 2nd Shot | 1st Shot | 2nd Shot | ||||||
| Vaccine Pfizer (Comirnaty) | |||||||||
| Vaccine Shot Schedule (n) | 87 | 87 | 23 | 22 | |||||
| Average dose 30 µg | |||||||||
| µg/kg | 0.417 | 0.417 | 0.47 | 0.47 | |||||
| µg/m2 body surface area | 16.36 | 16.36 | 17.56 | 17.56 | |||||
| Adverse events (n, % patients) * | |||||||||
| Local | Arm pain | 14 | (15.90%) | 12 | (13.63%) | 22 | (96.0%) | 12 | (52.0%) |
| Hematoma | 1 | (1.13%) | 2 | (2.27%) | 0 | (0.0%) | 0 | (0.00%) | |
| Systemic | Fatigue | 7 | (8.00%) | 4 | (4.54%) | 1 | (4.34%) | 7 | (30.43%) |
| Diarrhea | 2 | (2.27%) | 1 | (1.13%) | 0 | (0.00%) | 0 | (0.00%) | |
| Joint pain | 3 | (3.40%) | 1 | (1.13%) | 2 | (8.69%) | 2 | (8.69%) | |
| Fever | 6 | (7.0%) | 6 | (6.81%) | 4 | (17.39%) | 2 | (8.69%) | |
| Headache | 0 | (0.0%) | 0 | (0.00%) | 2 | (8.69%) | 1 | (4.34%) | |
| Vertigo | 1 | (1.13%) | 1 | (1.13%) | 0 | (0.00%) | 0 | (0.00%) | |
| Hospitalization ** | 1 | (1.13%) | 0 | (0.00%) | 0 | (0.00%) | 0 | (0.00%) | |
| No adverse event reported | 69 | (79.00%) | 65 | (73.86%) | 0 | (0.00%) | 8 | (34.78%) | |
| Total | 88 | 88 | 12 | 12 | |||||
| Baseline | 1st Shot | 2nd Shot | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Control Group | Patients | Control Group | Patients | Control Group | ||||
| Antibodies status before vaccination * | |||||||||
| IgG positive (% individuals) | 17.24% | 18% | |||||||
| IgM positive (% individuals) | 10.34% | 18% | |||||||
| Antibodies response to vaccination ** | |||||||||
| Anti-N (cutoff index) | |||||||||
| % individuals < 1 | 77.01% | 82.61% | 75.86% | 82.61% | |||||
| % individuals > 1 | 22.99% | 17.39% | 24.14% | 17.39% | |||||
| Anti-S titers (U/mL) | |||||||||
| % individuals < 0.4 | 4.6% | 0% | 2.3% | 0% | |||||
| % individuals 0.4–50 | 59.77% | 34.78% | 1.15% | 0% | |||||
| % individuals 50–250 | 11.49% | 39.13% | 2.3% | 4.35% | |||||
| % individuals > 250 | 24.14% | 26.09% | 94.25% | 95.65% | |||||
| Blood cells parameters | Mean | 95% CI § | Mean | 95% CI § | Mean | 95% CI § | |||
| Hemoglobin (g/dL) | 11.01 | 13.13 | (1.49, 2.75) | 11.26 | 13.47 | (1.6, 2.8) | 11.4 | 13.1 | (1.2, 2.2) |
| Platelets (n/mm3) | 222,207 | 253,391 | (−2305, 64,674) | 228,288 | 251,087 | (−12,349, 57,948) | 242,044 | 266,870 | (−36,296, 85,947) |
| White Blood Cells Count(n/mm3) | 6289.2 | 7163.0 | (−4.12, 1751.8) | 6178.3 | 7468.7 | (369, 2212) | 6001 | 7129.6 | (287, 1971) |
| Monocytes | 528.2 | 455.4 | (10.66, 134.96) | 527.1 | 453.5 | (−10.8, 158.0) | 525.7 | 494.4 | (−41.2, 103.9) |
| Lymphocytes | 1409.4 | 2435.6 | (673, 1379) | 1462.1 | 2500.7 | (506, 1571) | 1351.4 | 2968.9 | (1037, 2199) |
| % individuals < 1000/mm3 | 17.24% | 0.00% | 26.44% | 8.7% | 35.63% | 0.00% | |||
| Normovolemic (<+1.5 L) n = 49 | Mild Fluid Overload (1.5–3 L) n = 25 | Severe Fluid Overload (>3 L) n = 13 | |
|---|---|---|---|
| Fluid Overload (L) | Median (25th–75th percentile) | ||
| 0.49 (−0.34–0.95) | 2 (1.89–2.44) | 4.33 (3.64–4.99) | |
| Number of patients with urine output >300 mL/day | Number of patients (%) | ||
| 25 (51%) | 16 (64%) | 11 (85%) | |
| Dialysis conditions | Number of patients (% patients) | ||
| HD | 28 (57%) | 11 (44%) | 6 (46%) |
| HDF | 17 (35%) | 13 (52%) | 7 (54%) |
| Home Daily Hemodialysis | 4 (8%) | 1 (4%) | 0 (0%) |
| Dialysis performance | Median (25th–75th percentile) | ||
| Ultrafiltration Rate (mL) | 1760 (1200–2377) | 2000 (1617–2490) | 1800 (1485–2804) |
| Kt/V | 1.58 (1.3–1.85) | 1.59 (1.46–1.82) | 1.66 (1.39–1.8) |
| SARS-CoV-2 vaccine response | Median (25th–75th percentile) | ||
| Anti-S after 1st Shot (U/mL) | 23.9 (4.99–>250) | 11.54 (2.95–121.4) | 9.43 (1.59–22.42) |
| Anti-S after 2nd Shot (U/mL) | >250 (>250–>250) | >250 (>250–>250) | >250 (207.3–>250) |
| Levels of vaccine response * | Number of patients (% patients) | ||
| After 1st Shot: <50 U/mL | 28 (57%) | 17 (68%) | 11 (85%) |
| <250 U/mL | 35 (71%) | 19 (76%) | 13 (100%) |
| After 2nd Shot: <50 U/mL | 1 (2%) | 1 (4%) | 1 (8%) |
| <250 U/mL | 2 (4%) | 1 (4%) | 2 (15%) |
| Hepatitis B vaccine response | Number of patients (% patients) | ||
| Levels of vaccine response ** | |||
| Anti HBS < 10 IU/L | 6 (12%) | 3 (12%) | 1 (8%) |
| Anti HBS < 100 IU/L | 24 (49%) | 13 (52%) | 6 (46%) |
| Laboratory data | Median (25th–75th percentile) | ||
| Albumin | 41 (37–42) | 38 (36–40) | 39 (38–41) |
| C-Reactive Protein (CRP) | 6 (2.8–10.7) | 5.6 (3.5–9.9) | 10 (6–10.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hebibi, H.; Edeas, M.; Cornillac, L.; Beaudreuil, S.; Achiche, J.; Attaf, D.; Saibi, S.; Chazot, C.; Ouaaz, F.; Canaud, B. SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload. Kidney Dial. 2022, 2, 44-56. https://doi.org/10.3390/kidneydial2010006
Hebibi H, Edeas M, Cornillac L, Beaudreuil S, Achiche J, Attaf D, Saibi S, Chazot C, Ouaaz F, Canaud B. SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload. Kidney and Dialysis. 2022; 2(1):44-56. https://doi.org/10.3390/kidneydial2010006
Chicago/Turabian StyleHebibi, Hedia, Marvin Edeas, Laure Cornillac, Severine Beaudreuil, Jedjiga Achiche, David Attaf, Samah Saibi, Charles Chazot, Fatah Ouaaz, and Bernard Canaud. 2022. "SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload" Kidney and Dialysis 2, no. 1: 44-56. https://doi.org/10.3390/kidneydial2010006
APA StyleHebibi, H., Edeas, M., Cornillac, L., Beaudreuil, S., Achiche, J., Attaf, D., Saibi, S., Chazot, C., Ouaaz, F., & Canaud, B. (2022). SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload. Kidney and Dialysis, 2(1), 44-56. https://doi.org/10.3390/kidneydial2010006