Saccharomyces cerevisiae: Multifaceted Applications in One Health and the Achievement of Sustainable Development Goals
Definition
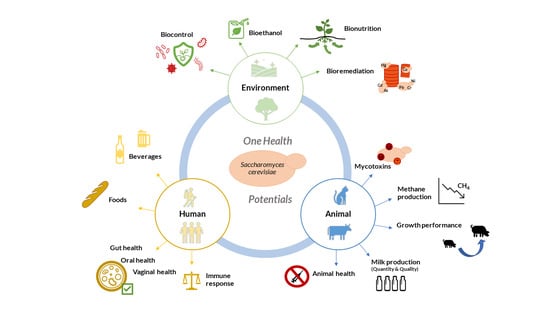
:1. Introduction
2. Plants and Environment
2.1. Agriculture: Yeast-Based Fertilizers and Biocontrol Agents
2.2. Environmental Benefits: Lowering Greenhouse Gas Emissions and Enhancing Bioremediation
3. Animals
3.1. Animal Health
3.2. Food Quality
4. Humans
4.1. Nutrition
4.2. SC to Improve Health
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/sites/default/files/publications/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 20 March 2023).
- Parapouli, M.; Vasileiadis, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Tomar, R.; Ganesan, K.; Prasad, G.S.; Subramanian, S. Complete genome sequence and comparative genomics of the probiotic yeast Saccharomyces boulardii. Sci. Rep. 2017, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Toh, M.; Liu, S.-Q. Growth, survival, and metabolic activities of probiotics Lactobacillus rhamnosus GG and Saccharomyces cerevisiae var. boulardii CNCM-I745 in fermented coffee brews. Int. J. Food Microbiol. 2021, 350, 109229. [Google Scholar] [CrossRef]
- Knight, S.J.; Goddard, M.R. Sporulation in soil as an overwinter survival strategy in Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, fov102. [Google Scholar] [CrossRef]
- Johnson, A.; Laney, K. (Eds.) Reducing the Health Impacts of the Nitrogen Problem: Proceedings of a Workshop—In Brief; National Academies Press: Washington, DC, USA, 2021; ISBN 0309094127. [Google Scholar]
- Locascio, A.; Andrés-Colás, N.; Mulet, J.M.; Yenush, L. Saccharomyces cerevisiae as a Tool to Investigate Plant Potassium and Sodium Transporters. Int. J. Mol. Sci. 2019, 20, 2133. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Mohamed, H. Molecular genetic identification of yeast strains isolated from egyptian soils for solubilization of inorganic phosphates and growth promotion of corn plants. J. Microbiol. Biotechnol. 2011, 21, 55–61. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Farran, I.; Larraya, L.; Ancin, M.; Arregui, L.M.; Veramendi, J. Plant growth-promoting traits of yeasts isolated from Spanish vineyards: Benefits for seedling development. Microbiol. Res. 2020, 237, 126480. [Google Scholar] [CrossRef]
- García-Gómez, A.; Bernal, M.P.; Roig, A. Carbon mineralisation and plant growth in soil amended with compost samples at different degrees of maturity. Waste Manag. Res. 2003, 21, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; López-Núñez, J.C.; Jones, M.A.; Moser, B.R.; Cox, E.J.; Lindquist, M.; Galindo-Leva, L.A.; Riaño-Herrera, N.M.; Rodriguez-Valencia, N.; Gast, F.; et al. Sustainable conversion of coffee and other crop wastes to biofuels and bioproducts using coupled biochemical and thermochemical processes in a multi-stage biorefinery concept. Appl. Microbiol. Biotechnol. 2014, 98, 8413–8431. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Shalaby, M.E.-S.; El-Nady, M.F. Application of Saccharomyces Cerevisiae as a Biocontrol Agent against Fusarium Infection of Sugar Beet Plants. Acta Biol. Szeged. 2008, 52, 271–275. [Google Scholar]
- Sui, Y.; Wisniewski, M.; Droby, S.; Liu, J. Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 2015, 81, 2968–2975. [Google Scholar] [CrossRef]
- Lucena, C.; Alcalá-Jiménez, M.T.; Romera, F.J.; Ramos, J. Several Yeast Species Induce Iron Deficiency Responses in Cucumber Plants (Cucumis sativus L.). Microorganisms 2021, 9, 2603. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Ferraz, P.; Brandão, R.L.; Cássio, F.; Lucas, C. Moniliophthora perniciosa, the Causal Agent of Cacao Witches’ Broom Disease Is Killed in vitro by Saccharomyces cerevisiae and Wickerhamomyces anomalus Yeasts. Front. Microbiol. 2021, 12, 706675. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Punja, Z.K.; Utkhede, R.S. Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol. 2003, 21, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sundh, I.; Wilcks, A.; Goettel, M.S. (Eds.) Beneficial Microorganisms in Agriculture, Food and the Environment: Safety Assessment and Regulation; CABI: Wallingford, CT, USA; Boston, MA, USA, 2012; ISBN 9781845938109. [Google Scholar]
- Cubaiu, L.; Abbas, H.; Dobson, A.D.W.; Budroni, M.; Migheli, Q. A Saccharomyces cerevisiae wine strain inhibits growth and decreases Ochratoxin A biosynthesis by Aspergillus carbonarius and Aspergillus ochraceus. Toxins 2012, 4, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Armando, M.R.; Pizzolitto, R.P.; Dogi, C.A.; Cristofolini, A.; Merkis, C.; Poloni, V.; Dalcero, A.M.; Cavaglieri, L.R. Adsorption of ochratoxin A and zearalenone by potential probiotic Saccharomyces cerevisiae strains and its relation with cell wall thickness. J. Appl. Microbiol. 2012, 113, 256–264. [Google Scholar] [CrossRef]
- Armando, M.R.; Dogi, C.A.; Poloni, V.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. In vitro study on the effect of Saccharomyces cerevisiae strains on growth and mycotoxin production by Aspergillus carbonarius and Fusarium graminearum. Int. J. Food Microbiol. 2013, 161, 182–188. [Google Scholar] [CrossRef]
- Armando, M.R.; Dogi, C.A.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Saccharomyces cerevisiae strains and the reduction of Aspergillus parasiticus growth and aflatoxin B1 production at different interacting environmental conditions, in vitro. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1443–1449. [Google Scholar] [CrossRef]
- Ekwomadu, T.; Mwanza, M.; Musekiwa, A. Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue. Int. J. Environ. Res. Public Health 2022, 19, 7754. [Google Scholar] [CrossRef] [PubMed]
- Oporto, C.I.; Villarroel, C.A.; Tapia, S.M.; García, V.; Cubillos, F.A. Distinct Transcriptional Changes in Response to Patulin Underlie Toxin Biosorption Differences in Saccharomyces Cerevisiae. Toxins 2019, 11, 400. [Google Scholar] [CrossRef]
- Faucet-Marquis, V.; Joannis-Cassan, C.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Development of an in vitro method for the prediction of mycotoxin binding on yeast-based products: Case of aflatoxin B1, zearalenone and ochratoxin A. Appl. Microbiol. Biotechnol. 2014, 98, 7583–7596. [Google Scholar] [CrossRef]
- Joannis-Cassan, C.; Tozlovanu, M.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Binding of zearalenone, aflatoxin B1, and ochratoxin A by yeast-based products: A method for quantification of adsorption performance. J. Food Prot. 2011, 74, 1175–1185. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Marques, W.L.; Raghavendran, V.; Stambuk, B.U.; Gombert, A.K. Sucrose and Saccharomyces cerevisiae: A relationship most sweet. FEMS Yeast Res. 2016, 16, fov107. [Google Scholar] [CrossRef]
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, e1800421. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.L.; Costa, C.E.; Cunha, J.T.; Soares, P.O.; Domingues, L. Metabolic engineering of Saccharomyces cerevisiae for the production of top value chemicals from biorefinery carbohydrates. Biotechnol. Adv. 2021, 47, 107697. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Soares, P.O.; Baptista, S.L.; Costa, C.E.; Domingues, L. Engineered Saccharomyces cerevisiae for lignocellulosic valorization: A review and perspectives on bioethanol production. Bioengineered 2020, 11, 883–903. [Google Scholar] [CrossRef] [PubMed]
- Cámara, E.; Olsson, L.; Zrimec, J.; Zelezniak, A.; Geijer, C.; Nygård, Y. Data mining of Saccharomyces cerevisiae mutants engineered for increased tolerance towards inhibitors in lignocellulosic hydrolysates. Biotechnol. Adv. 2022, 57, 107947. [Google Scholar] [CrossRef]
- Chen, Y. Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: A systematic review. J. Ind. Microbiol. Biotechnol. 2011, 38, 581–597. [Google Scholar] [CrossRef]
- Massoud, R.; Zoghi, A. Potential probiotic strains with heavy metals and mycotoxins bioremoval capacity for application in foodstuffs. J. Appl. Microbiol. 2022, 133, 1288–1307. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Sharifan, A.; Asadi, G.H. Lead bioremoval from milk by Saccharomyces cerevisiae. Biocatal. Agric. Biotechnol. 2019, 22, 101437. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef]
- Damodaran, D.; Suresh, G.; Mohan, R. Bioremediation of soil by removing heavy metals using Saccharomyces cerevisiae. In Proceedings of the 2nd International Conference on Environmental Science and Technology, Singapore, 26–28 February 2011. [Google Scholar]
- Chen, B.; Lee, H.L.; Heng, Y.C.; Chua, N.; Teo, W.S.; Choi, W.J.; Leong, S.S.J.; Foo, J.L.; Chang, M.W. Synthetic biology toolkits and applications in Saccharomyces cerevisiae. Biotechnol. Adv. 2018, 36, 1870–1881. [Google Scholar] [CrossRef]
- Massoud, R.; Sharifan, A.; Khosravi-Darani, K.; Asadi, G. Cadmium Bioremoval by Saccharomyces cerevisiae in Milk. JoMMID 2020, 8, 29–33. [Google Scholar] [CrossRef]
- Hadiani, M.R.; Khosravi-Darani, K.; Rahimifard, N.; Younesi, H. Assessment of Mercury biosorption by Saccharomyces cerevisiae: Response surface methodology for optimization of low Hg (II) concentrations. J. Environ. Chem. Eng. 2018, 6, 4980–4987. [Google Scholar] [CrossRef]
- Ozer, A.; Ozer, D. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: Determination of biosorption heats. J. Hazard. Mater. 2003, 100, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, M.; Yang, Q.; Apaliya, M.T.; Li, J.; Zhang, X. Biodegradation of zearalenone by Saccharomyces cerevisiae: Possible involvement of ZEN responsive proteins of the yeast. J. Proteomics 2016, 143, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Abrunhosa, L.; Keller, K.; Rosa, C.A.; Cavaglieri, L.; Venâncio, A. Zearalenone and Its Derivatives α-Zearalenol and β-Zearalenol Decontamination by Saccharomyces cerevisiae Strains Isolated from Bovine Forage. Toxins 2015, 7, 3297–3308. [Google Scholar] [CrossRef]
- Geva, P.; Kahta, R.; Nakonechny, F.; Aronov, S.; Nisnevitch, M. Increased copper bioremediation ability of new transgenic and adapted Saccharomyces cerevisiae strains. Environ. Sci. Pollut. Res. Int. 2016, 23, 19613–19625. [Google Scholar] [CrossRef] [PubMed]
- Darabighane, B.; Salem, A.Z.M.; Mirzaei Aghjehgheshlagh, F.; Mahdavi, A.; Zarei, A.; Elghandour, M.M.M.Y.; López, S. Environmental efficiency of Saccharomyces cerevisiae on methane production in dairy and beef cattle via a meta-analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 3651–3658. [Google Scholar] [CrossRef]
- Hernández, A.; Kholif, A.E.; Elghandour, M.M.; Camacho, L.M.; Cipriano, M.M.; Salem, A.Z.; Cruz, H.; Ugbogu, E.A. Effectiveness of xylanase and Saccharomyces cerevisiae as feed additives on gas emissions from agricultural calf farms. J. Clean. Prod. 2017, 148, 616–623. [Google Scholar] [CrossRef]
- Burdick Sanchez, N.C.; Broadway, P.R.; Carroll, J.A. Influence of Yeast Products on Modulating Metabolism and Immunity in Cattle and Swine. Animals 2021, 11, 371. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Yu, Z.; Zhou, G.; Yao, J. Effects of supplementation with Saccharomyces cerevisiae products on dairy calves: A meta-analysis. J. Dairy Sci. 2022, 105, 7386–7398. [Google Scholar] [CrossRef]
- Villot, C.; Ma, T.; Renaud, D.L.; Ghaffari, M.H.; Gibson, D.J.; Skidmore, A.; Chevaux, E.; Guan, L.L.; Steele, M.A. Saccharomyces cerevisiae boulardii CNCM I-1079 affects health, growth, and fecal microbiota in milk-fed veal calves. J. Dairy Sci. 2019, 102, 7011–7025. [Google Scholar] [CrossRef] [PubMed]
- Perricone, V.; Sandrini, S.; Irshad, N.; Savoini, G.; Comi, M.; Agazzi, A. Yeast-Derived Products: The Role of Hydrolyzed Yeast and Yeast Culture in Poultry Nutrition-A Review. Animals 2022, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Xia, R.; Zhang, Q.; Xie, Y.; Ran, C.; Yang, Y.; Zhou, W.; Chu, F.; Zhang, X.; Wang, Y.; et al. Partially replacing dietary fish meal by Saccharomyces cerevisiae culture improve growth performance, immunity, disease resistance, composition and function of intestinal microbiota in channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2022, 125, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Esteban, M.Á. Effect of dietary supplementation with yeast Saccharomyces cerevisiae on skin, serum and liver of gilthead seabream (Sparus aurata L.). J. Fish Biol. 2020, 97, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Cheng, C.-H.; Gua, W.-R.; Guu, Y.-K.; Cheng, W. Dietary administration of the probiotic, Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol. 2010, 29, 1053–1059. [Google Scholar] [CrossRef]
- Goddard, M.R.; Greig, D. Saccharomyces cerevisiae: A nomadic yeast with no niche? FEMS Yeast Res. 2015, 15, fov009. [Google Scholar] [CrossRef]
- Meriggi, N.; Di Paola, M.; Cavalieri, D.; Stefanini, I. Saccharomyces cerevisiae–Insects Association: Impacts, Biogeography, and Extent. Front. Microbiol. 2020, 11, 1629. [Google Scholar] [CrossRef]
- Benato, L.; Hastie, P.; O’Shaughnessy, P.; Murray, J.-A.; Meredith, A. Effects of probiotic Enterococcus faecium and Saccharomyces cerevisiae on the faecal microflora of pet rabbits. J. Small Anim. Pract. 2014, 55, 442–446. [Google Scholar] [CrossRef]
- de Oliveira Matheus, L.F.; Risolia, L.W.; Ernandes, M.C.; de Souza, J.M.; Oba, P.M.; Vendramini, T.H.A.; Pedrinelli, V.; Henríquez, L.B.F.; de Oliveira Massoco, C.; Pontieri, C.F.F.; et al. Effects of Saccharomyces cerevisiae cell wall addition on feed digestibility, fecal fermentation and microbiota and immunological parameters in adult cats. BMC Vet. Res. 2021, 17, 351. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.C.; Weaver, D.M.; Yiannikouris, A.; Adams, N. Meta-analysis of the effects of mycotoxins and yeast cell wall extract supplementation on the performance, livability, and environmental sustainability of broiler production. Poult. Sci. 2022, 101, 102043. [Google Scholar] [CrossRef] [PubMed]
- Badia, R.; Brufau, M.T.; Guerrero-Zamora, A.M.; Lizardo, R.; Dobrescu, I.; Martin-Venegas, R.; Ferrer, R.; Salmon, H.; Martínez, P.; Brufau, J. β-Galactomannan and Saccharomyces cerevisiae var. boulardii Modulate the Immune Response against Salmonella enterica Serovar Typhimurium in Porcine Intestinal Epithelial and Dendritic Cells. Clin. Vaccine Immunol. 2012, 19, 368–376. [Google Scholar] [CrossRef]
- Zanello, G.; Meurens, F.; Berri, M.; Chevaleyre, C.; Melo, S.; Auclair, E.; Salmon, H. Saccharomyces cerevisiae decreases inflammatory responses induced by F4+ enterotoxigenic Escherichia coli in porcine intestinal epithelial cells. Vet. Immunol. Immunopathol. 2011, 141, 133–138. [Google Scholar] [CrossRef]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef]
- Fadl, S.E.; Elbialy, Z.I.; Abdo, W.; Saad, A.H.; Aboubakr, M.; Abdeen, A.; Elkamshishi, M.M.; Salah, A.S.; El-Mleeh, A.; Almeer, R.; et al. Ameliorative effect of Spirulina and Saccharomyces cerevisiae against fipronil toxicity in Oreochromis niloticus. Ecotoxicol. Environ. Saf. 2022, 242, 113899. [Google Scholar] [CrossRef]
- Protein in diet: MedlinePlus Medical Encyclopedia. 2021. Available online: https://medlineplus.gov/ency/article/002467.htm (accessed on 20 March 2023).
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Dardelle, G.; Normand, V.; Steenhoudt, M.; Bouquerand, P.E.; Chevalier, M.; Baumgartner, P. Flavour-encapsulation and flavour-release performances of a commercial yeast-based delivery system. Food Hydrocoll. 2007, 21, 953–960. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Shojaee-Aliabadi, S.; Dadkhodazade, E.; Sheidaei, Z.; Abedi, A.-S.; Mirmoghtadaie, L.; Hosseini, S.M. Comparison of Properties of Breads Enriched with Omega-3 Oil Encapsulated in β-Glucan and Saccharomyces cerevisiae Yeast Cells. Appl. Food Biotechnol. 2019, 7, 11–20. [Google Scholar] [CrossRef]
- Dadkhodazade, E.; Khanniri, E.; Khorshidian, N.; Hosseini, S.M.; Mortazavian, A.M.; Moghaddas Kia, E. Yeast cells for encapsulation of bioactive compounds in food products: A review. Biotechnol. Prog. 2021, 37, e3138. [Google Scholar] [CrossRef] [PubMed]
- Paramera, E.I.; Karathanos, V.T.; Konteles, S.J. Yeast Cells and Yeast-Based Materials for Microencapsulation. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; pp. 267–281. ISBN 9780124045682. [Google Scholar]
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef] [PubMed]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Ind. Crops Prod. 2013, 42, 469–479. [Google Scholar] [CrossRef]
- Ruphuy, G.; Saloň, I.; Tomas, J.; Šalamúnová, P.; Hanuš, J.; Štěpánek, F. Encapsulation of poorly soluble drugs in yeast glucan particles by spray drying improves dispersion and dissolution properties. Int. J. Pharm. 2020, 576, 118990. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhou, Y.J.; Krivoruchko, A.; Huang, M.; Liu, L.; Khoomrung, S.; Siewers, V.; Jiang, B.; Nielsen, J. Modular pathway rewiring of Saccharomyces cerevisiae enables high-level production of L-ornithine. Nat. Commun. 2015, 6, 8224. [Google Scholar] [CrossRef]
- Koopman, F.; Beekwilder, J.; Crimi, B.; van Houwelingen, A.; Hall, R.D.; Bosch, D.; van Maris, A.J.A.; Pronk, J.T.; Daran, J.-M. De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb. Cell Fact. 2012, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Consoli, M.L.D.; Da Silva, R.S.; Nicoli, J.R.; Bruña-Romero, O.; Da Silva, R.G.; de Vasconcelos Generoso, S.; Correia, M.I.T.D. Randomized Clinical Trial: Impact of Oral Administration of Saccharomyces boulardii on Gene Expression of Intestinal Cytokines in Patients Undergoing Colon Resection. JPEN J. Parenter. Enteral Nutr. 2016, 40, 1114–1121. [Google Scholar] [CrossRef]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef]
- Roussel, C.; de Paepe, K.; Galia, W.; de Bodt, J.; Chalancon, S.; Denis, S.; Leriche, F.; Vandekerkove, P.; Ballet, N.; Blanquet-Diot, S.; et al. Multi-targeted properties of the probiotic saccharomyces cerevisiae CNCM I-3856 against enterotoxigenic escherichia coli (ETEC) H10407 pathogenesis across human gut models. Gut Microbes 2021, 13, 1953246. [Google Scholar] [CrossRef]
- Cayzeele-Decherf, A.; Pélerin, F.; Leuillet, S.; Douillard, B.; Housez, B.; Cazaubiel, M.; Jacobson, G.K.; Jüsten, P.; Desreumaux, P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J. Gastroenterol. 2017, 23, 336–344. [Google Scholar] [CrossRef]
- Mourey, F.; Decherf, A.; Jeanne, J.-F.; Clément-Ziza, M.; Grisoni, M.-L.; Machuron, F.; Legrain-Raspaud, S.; Bourreille, A.; Desreumaux, P. Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation. World J. Gastroenterol. 2022, 28, 2509–2522. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Sivignon, A.; de Vallée, A.; Barnich, N.; Denizot, J.; Darcha, C.; Pignède, G.; Vandekerckove, P.; Darfeuille-Michaud, A. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 276–286. [Google Scholar] [CrossRef]
- Garcia Vilela, E.; de Lourdes Abreu Ferrari, M.; Da Oswaldo Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.; Da Sales Cunha, A. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Guslandi, M.; Mezzi, G.; Sorghi, M.; Testoni, P.A. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig. Dis. Sci. 2000, 45, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Bourreille, A.; Cadiot, G.; Le Dreau, G.; Laharie, D.; Beaugerie, L.; Dupas, J.-L.; Marteau, P.; Rampal, P.; Moyse, D.; Saleh, A.; et al. Saccharomyces boulardii does not prevent relapse of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2013, 11, 982–987. [Google Scholar] [CrossRef]
- Sabbatini, S.; Monari, C.; Ballet, N.; Cayzeele Decherf, A.; Bozza, S.; Camilloni, B.; Perito, S.; Vecchiarelli, A. Anti-Biofilm Properties of Saccharomyces cerevisiae CNCM I-3856 and Lacticaseibacillus rhamnosus ATCC 53103 Probiotics against G. vaginalis. Microorganisms 2020, 8, 1294. [Google Scholar] [CrossRef]
- Sabbatini, S.; Monari, C.; Ballet, N.; Mosci, P.; Cayzeele Decherf, A.; Pélerin, F.; Perito, S.; Scarpelli, P.; Vecchiarelli, A. Saccharomyces cerevisiae-based probiotic as novel anti-microbial agent for therapy of bacterial vaginosis. Virulence 2018, 9, 954–966. [Google Scholar] [CrossRef]
- Oerlemans, E.; Ahannach, S.; Wittouck, S.; Dehay, E.; de Boeck, I.; Ballet, N.; Rodriguez, B.; Tuyaerts, I.; Lebeer, S. Impacts of Menstruation, Community Type, and an Oral Yeast Probiotic on the Vaginal Microbiome. mSphere 2022, 7, e0023922. [Google Scholar] [CrossRef]
- Roselletti, E.; Sabbatini, S.; Ballet, N.; Perito, S.; Pericolini, E.; Blasi, E.; Mosci, P.; Cayzeele Decherf, A.; Monari, C.; Vecchiarelli, A. Saccharomyces cerevisiae CNCM I-3856 as a New Therapeutic Agent Against Oropharyngeal Candidiasis. Front. Microbiol. 2019, 10, 1469. [Google Scholar] [CrossRef]
- Gabrielli, E.; Pericolini, E.; Ballet, N.; Roselletti, E.; Sabbatini, S.; Mosci, P.; Cayzeele Decherf, A.; Pélerin, F.; Perito, S.; Jüsten, P.; et al. Saccharomyces cerevisiae-based probiotic as novel anti-fungal and anti-inflammatory agent for therapy of vaginal candidiasis. Benef. Microbes 2018, 9, 219–230. [Google Scholar] [CrossRef]
- Pericolini, E.; Gabrielli, E.; Ballet, N.; Sabbatini, S.; Roselletti, E.; Cayzeele Decherf, A.; Pélerin, F.; Luciano, E.; Perito, S.; Jüsten, P.; et al. Therapeutic activity of a Saccharomyces cerevisiae-based probiotic and inactivated whole yeast on vaginal candidiasis. Virulence 2017, 8, 74–90. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballet, N.; Renaud, S.; Roume, H.; George, F.; Vandekerckove, P.; Boyer, M.; Durand-Dubief, M. Saccharomyces cerevisiae: Multifaceted Applications in One Health and the Achievement of Sustainable Development Goals. Encyclopedia 2023, 3, 602-613. https://doi.org/10.3390/encyclopedia3020043
Ballet N, Renaud S, Roume H, George F, Vandekerckove P, Boyer M, Durand-Dubief M. Saccharomyces cerevisiae: Multifaceted Applications in One Health and the Achievement of Sustainable Development Goals. Encyclopedia. 2023; 3(2):602-613. https://doi.org/10.3390/encyclopedia3020043
Chicago/Turabian StyleBallet, Nathalie, Sarah Renaud, Hugo Roume, Fanny George, Pascal Vandekerckove, Mickaël Boyer, and Mickaël Durand-Dubief. 2023. "Saccharomyces cerevisiae: Multifaceted Applications in One Health and the Achievement of Sustainable Development Goals" Encyclopedia 3, no. 2: 602-613. https://doi.org/10.3390/encyclopedia3020043
APA StyleBallet, N., Renaud, S., Roume, H., George, F., Vandekerckove, P., Boyer, M., & Durand-Dubief, M. (2023). Saccharomyces cerevisiae: Multifaceted Applications in One Health and the Achievement of Sustainable Development Goals. Encyclopedia, 3(2), 602-613. https://doi.org/10.3390/encyclopedia3020043