Stem Cells and Cancer Therapeutics
(Closed)
Share This Topical Collection
Editors
 Dr. Lluis Espinosa
Dr. Lluis Espinosa
 Dr. Lluis Espinosa
Dr. Lluis Espinosa
E-Mail
Website
Collection Editor
Stem Cells and Cancer Research Laboratory, CIBERONC. Institut Hospital del Mar Investigacions Mèdiques (IMIM), 08003 Barcelona, Spain
Interests: signaling pathways; NF-kappaB; Notch; intestinal stem cells; colorectal cancer
Special Issues, Collections and Topics in MDPI journals
 Dr. Teresa Lobo-Jarne
Dr. Teresa Lobo-Jarne
 Dr. Teresa Lobo-Jarne
Dr. Teresa Lobo-Jarne
E-Mail
Website
Guest Editor
Stem Cells and Cancer Research Laboratory, CIBERONC. Institut Hospital del Mar Investigacions Mèdiques (IMIM), 08003 Barcelona, Spain
Interests: cancer metabolism; colorectal cancer; therapy resistance; patient-derived organoids; big data analysis
Topical Collection Information
Dear Colleagues,
Stems cells are defined as cells with the ability to self-renew and to differentiate into multiple lineages. This definition applies to both physiologic and pathologic conditions such as cancer, although functional differences in these cell populations crucially impact on their behavior. In the case of cancer, multiple experimental pieces of evidence support the existence of specific cell populations that are functionally identified for their capacity to initiate and propagate tumors. These cells have been classically called tumor initiating cells (TICs) or cancer stem cells (CSCs) and are supposed to possess stem cell-like properties, including long-term self-renewal, capacity of multi-lineage differentiation, increased resistance to therapy, and the ability to promote tumor relapse and metastasis. As in normal stem cells, essential developmental related pathways such as Wnt, Notch, Hedgehog, NF-kappaB, or JAK/STAT participate in supporting TIC capacity. This Special Issue will focus on understanding the molecular mechanisms that control normal and cancer-related stemness and the tools that are currently available for studying them both in vitro and in vivo. In particular, we will pay attention to how TICs contribute to cancer progression and therapy resistance, and the possibility of specifically targeting TIC in anti-cancer therapies.
Dr. Lluis Espinosa
Dr. Anna Vert
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomedicines is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Signaling pathways in normal and tumor stem cells
- Cancer stem cells and their biology
- Stem cells and metastasis
- In vivo models for studying normal and cancer stem cells
- In vitro models for studying normal and cancer stem cells
- Targeting cancer stem cells
Related Special Issue
Published Papers (2 papers)
Open AccessReview
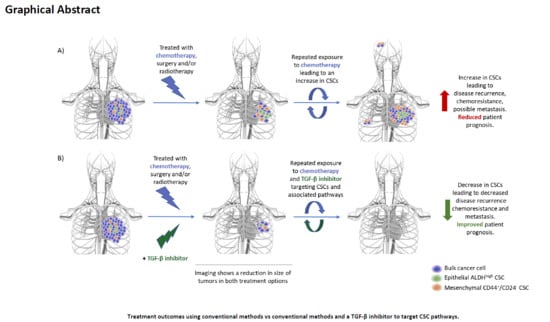
Clinically Translatable Approaches of Inhibiting TGF-β to Target Cancer Stem Cells in TNBC
by
Andrew Sulaiman, Sarah McGarry, Sai Charan Chilumula, Rohith Kandunuri and Vishak Vinod
Cited by 16 | Viewed by 3980
Abstract
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that disproportionally accounts for the majority of breast cancer-related deaths due to the lack of specific targets for effective treatments. In this review, we highlight the complexity of the transforming growth factor-beta family
[...] Read more.
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that disproportionally accounts for the majority of breast cancer-related deaths due to the lack of specific targets for effective treatments. In this review, we highlight the complexity of the transforming growth factor-beta family (TGF-β) pathway and discuss how the dysregulation of the TGF-β pathway promotes oncogenic attributes in TNBC, which negatively affects patient prognosis. Moreover, we discuss recent findings highlighting TGF-β inhibition as a potent method to target mesenchymal (CD44
+/CD24
−) and epithelial (ALDH
high) cancer stem cell (CSC) populations. CSCs are associated with tumorigenesis, metastasis, relapse, resistance, and diminished patient prognosis; however, due to differential signal pathway enrichment and plasticity, these populations remain difficult to target and persist as a major barrier barring successful therapy. This review highlights the importance of TGF-β as a driver of chemoresistance, radioresistance and reduced patient prognosis in breast cancer and highlights novel treatment strategies which modulate TGF-β, impede cancer progression and reduce the rate of resistance generation via targeting the CSC populations in TNBC and thus reducing tumorigenicity. Potential TGF-β inhibitors targeting based on clinical trials are summarized for further investigation, which may lead to the development of novel therapies to improve TNBC patient prognosis.
Full article
►▼
Show Figures
Open AccessArticle
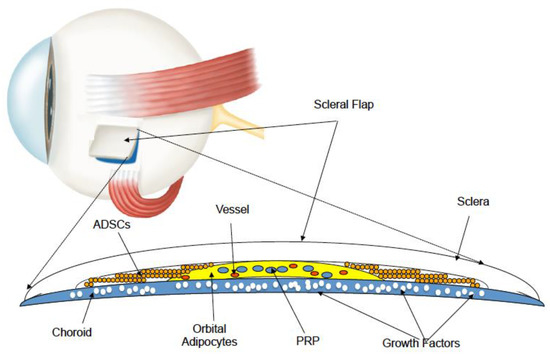
Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review
by
Paolo Giuseppe Limoli, Enzo Maria Vingolo, Celeste Limoli and Marcella Nebbioso
Cited by 15 | Viewed by 4172
Abstract
To evaluate whether grafting of autologous mesenchymal cells, adipose-derived stem cells, and platelet-rich plasma into the supracoroideal space by surgical treatment with the Limoli retinal restoration technique (LRRT) can exert a beneficial effect in retinitis pigmentosa (RP) patients. Twenty-one eyes underwent surgery and
[...] Read more.
To evaluate whether grafting of autologous mesenchymal cells, adipose-derived stem cells, and platelet-rich plasma into the supracoroideal space by surgical treatment with the Limoli retinal restoration technique (LRRT) can exert a beneficial effect in retinitis pigmentosa (RP) patients. Twenty-one eyes underwent surgery and were divided based on retinal foveal thickness (FT) ≤ 190 or > 190 µm into group A-FT and group B-FT, respectively. The specific LRRT triad was grafted in a deep scleral pocket above the choroid of each eye. At 6-month follow-up, group B showed a non-significant improvement in residual close-up visus and sensitivity at microperimetry compared to group A. After an in-depth review of molecular biology studies concerning degenerative phenomena underlying the etiopathogenesis of retinitis pigmentosa (RP), it was concluded that further research is needed on tapeto-retinal degenerations, both from a clinical and molecular point of view, to obtain better functional results. In particular, it is necessary to increase the number of patients, extend observation timeframes, and treat subjects in the presence of still trophic retinal tissue to allow adequate biochemical and functional catering.
Full article
►▼
Show Figures