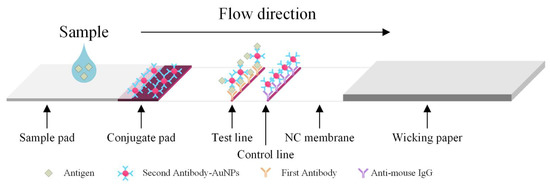
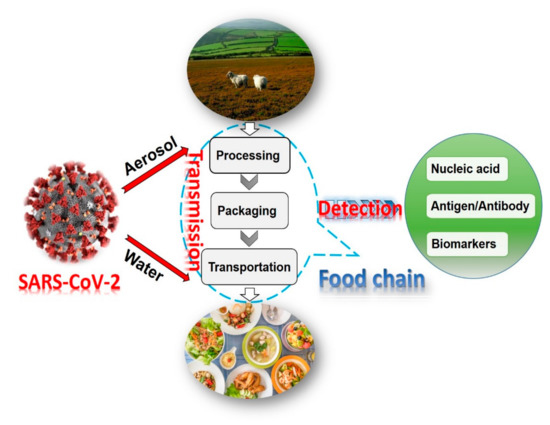
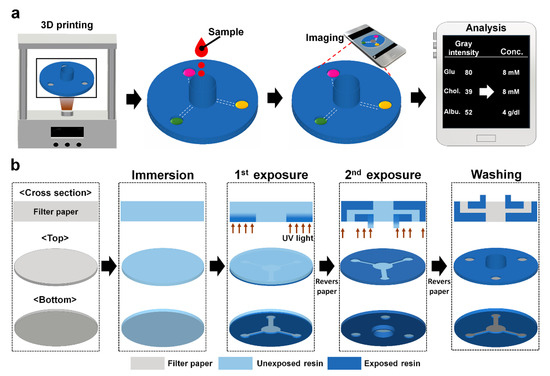
Biosensors for Point-of-Care Diagnostics (Closed)
A topical collection in Biosensors (ISSN 2079-6374).
Viewed by 37549Editor
2. Graduate School of Biomedical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
Interests: biosensors; point-of-care diagnostics; microfluidic-paper-based analytical devices µPADs; intelligent nanoparticles; medical devices
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear colleagues,
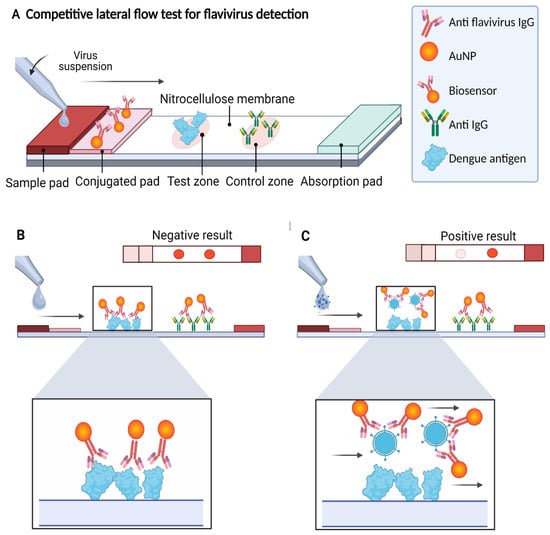
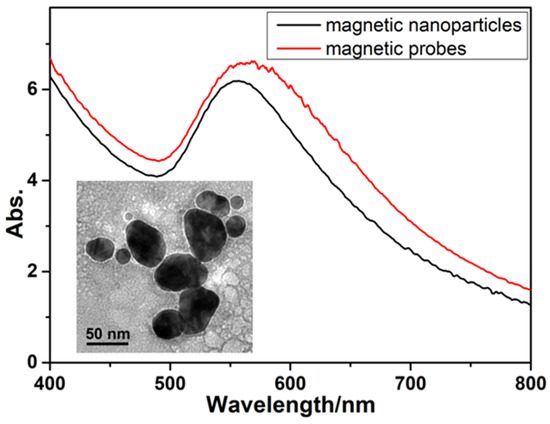
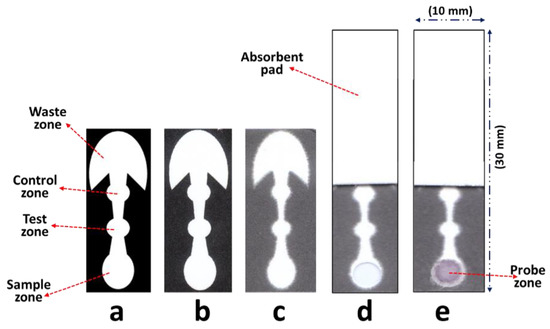
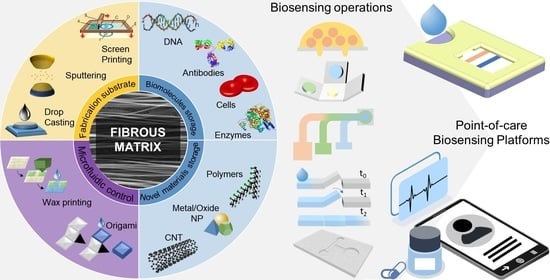
Diagnostics plays an essential role in the healthcare realm. The reliability and accuracy of diagnostics has a great impact on clinical decision-making, treatment, and patient survival rate. Infectious diseases such as immunodeficiency syndrome (AIDS), tuberculosis (TB), and the current COVID-19 pandemic, and different types of cancer cause a huge burden to the economy, especially in developing countries due to the low survival rate and the cause of disability. Additionally, the lack of laboratories and modern equipment makes it difficult for patients in rural areas to be treated fast and wisely. The current diagnostic technologies, such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), etc., require trained personnel and expensive instruments, which are not suitable for point-of-care (POC) diagnostics, especially in resource-limited settings. POC diagnostic technologies (such as paper lateral flow assay, disposbaible biosensors, µPADs, wearable biosensors, etc.) provide rapid testing at or near patients and are attractive in healthcare delivery due to their fast, user-friendly, and cost-efficient characteristics. The global market of POC diagnostics was valued at USD23.71 Billion in 2017 and is estimated to increase to USD38.13 Billion by 2022, with a compound annual growth rate (CAGR) of 10% during the forecast period.
Prof. Dr. Guozhen Liu
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biosensors is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- point-of-care diagnostics
- biosensors
- microfluidic-paper-based analytical devices
- paper lateral flow assay
- smartphone-based diagnostics
- disposable biosensors
- microfluidic sensing chips
- printable biosensors
- werable biosensors
- biomarker deteciton