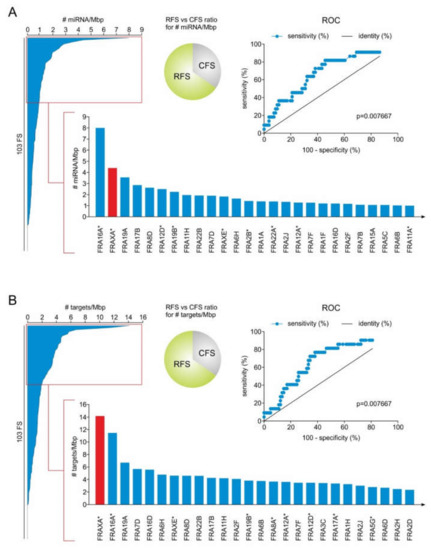
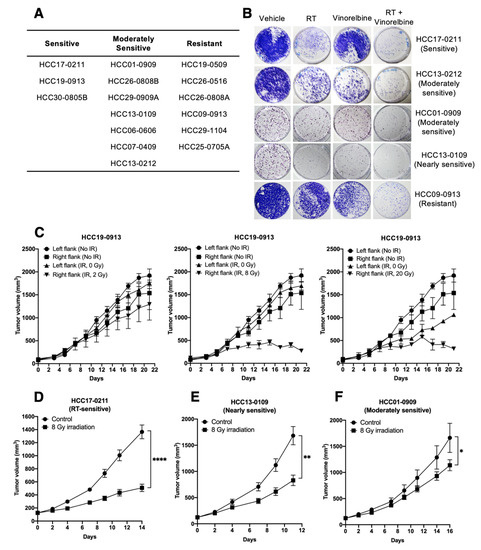
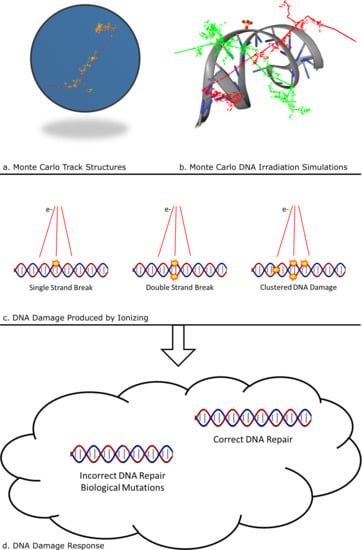
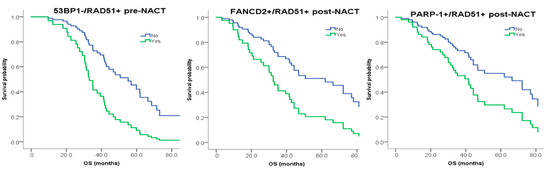
Incorporating DNA Damage Response (DDR) Mechanisms in Cancer Systems (Closed)
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Molecular Cancer Biology".
Viewed by 104676Editor
Interests: radiation biology; cancer biology; DNA damage and repair; oxidative stress; carcinogenesis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The chronic exposure of cells and organisms to exogenous (environmental) or endogenous stress can lead to the deterioration of the defense and homeostatic mechanisms, like antioxidants, DNA damage response (DDR), and immune response, which are strongly associated. This phenomenon is always fueled by the increasing and persistent genomic instability, which is considered to be one of the major detrimental effects of the different types of stresses originating from ionizing and non-ionizing radiations, replication problems, and others. From simple cellular systems to complex biological systems like tissues or organs, it is important to know how each system responds to DNA damage, especially of a complex type, and what are the final short- and long-terms effects?
Authors are invited to submit manuscripts dealing with the mechanisms or phenomena that can lead to any type of DDR induction in any biological system, or even method papers for the proper measurement of DDR and its biological outcome. We live in the era of omics and big data, therefore, teams working in this field by means of bioinformatics, systems biology, and machine learning, as well as any type of omics, are highly welcome.
In this Topical Collection, we are reaching out for teams or groups working in many interdisciplinary fields towards a better understanding of the complexity of cancer evolution, especially the role of malfunctioning DDR and its repair. We also expect contributions from groups working on the clinical aspects of DDR and its role in the regulation of homeostasis in the organism.
Prof. Dr. Alexandros Georgakilas
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Complex DNA damage
- Methodologies for detection of DNA damage
- DNA damage response (DDR) and repair
- Biological effects of radiation stress, oxidative stress, and replication stress
- DDR and immune response
- Genomic instability
- Aging
- Bioinformatics and systems biology
- Machine learning and artificial intelligence
- Cancer survival and therapeutics
- 3D bioprinting of cells and tissues