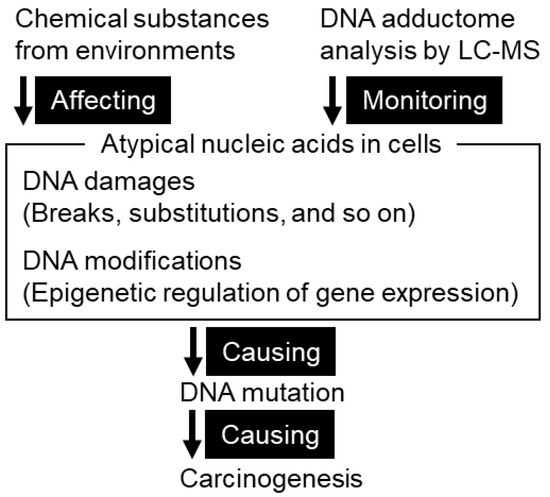
Carcinogenesis Model
(Closed)
Share This Topical Collection
Editor
 Dr. Yoshitaka Hippo
Dr. Yoshitaka Hippo
 Dr. Yoshitaka Hippo
Dr. Yoshitaka Hippo
E-Mail
Website
Collection Editor
Division of Molecular Carcinogenesis, Chiba Cancer Center Research Institute, 666-2, Nitona-cho, Chuo-ku, Chiba-city, Chiba 260-8717, Japan
Interests: multi-step carcinogenesis; animal models; genetic engineering; cellular transformation; organoids; preclinical models; patient-derived cells
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Accumulated genetic alterations can initiate and drive carcinogenesis, which are subjected to promotion by epigenetic changes through interactions with many different types of cells in the tissue microenvironment. By genetic engineering and/or chemical treatments, researchers have created many in vivo animal models, which successfully recapitulated multistep carcinogenesis from normal cells to premalignant lesions, full-blown tumors and metastases. They have not only contributed to deepening our understanding of the mechanisms underlying carcinogenesis, but also allowed us to investigate genes and compounds that could potentially affect development or maintenance of tumors. More recently, genome-editing technologies significantly accelerated generation of mutant animals, let alone mice, and organoid-based ex vivo carcinogenesis models have been also documented by us and other researchers. In light of such rapid advances in the field of carcinogenesis models, we need to put together various different types of models -including those generated by conventional or novel methods- to further promote cancer research. In this Topical Collection of Cancers, we welcome original research articles or comprehensive review articles focusing on development of, or by using, any types of models reflecting any steps of carcinogenesis, in any species or organs. I hope that such collection of studies will further increase our knowledge on carcinogenesis and provide powerful tools to fight against cancer.
Dr. Yoshitaka Hippo
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- tumorigenesis
- models
- genetically engineered mouse (GEM)
- chemical carcinogenesis
- cellular transformation
- organoids
- pre-cancerous lesions
- metastasis
- microenvironments
Published Papers (15 papers)
Open AccessReview
Geospatial Assessments of DNA Adducts in the Human Stomach: A Model of Field Cancerization
by
Yuji Iwashita, Ippei Ohnishi, Yuto Matsushita, Shunsuke Ohtsuka, Takashi Yamashita, Keisuke Inaba, Atsuko Fukazawa, Hideto Ochiai, Keigo Matsumoto, Nobuhito Kurono, Yoshitaka Matsushima, Hiroki Mori, Shioto Suzuki, Shohachi Suzuki, Fumihiko Tanioka and Haruhiko Sugimura
Cited by 5 | Viewed by 2293
Abstract
Background: Field cancerization is a popular concept regarding where cancer cells arise in a plane, such as the opened-up gastrointestinal mucosa. The geospatial distribution of DNA adducts, some of which are believed to initiate mutation, may be a clue to understanding the landscape
[...] Read more.
Background: Field cancerization is a popular concept regarding where cancer cells arise in a plane, such as the opened-up gastrointestinal mucosa. The geospatial distribution of DNA adducts, some of which are believed to initiate mutation, may be a clue to understanding the landscape of the preferred occurrence of gastric cancer in the human stomach, such that the occurrence is much more frequent in the lesser curvature than in the greater curvature. Methods: Seven DNA adducts, C5-methyl-2′-deoxycytidine, 2′-deoxyinosine, C5-hydroxymethyl-2′-deoxycytidine, N6-methyl-2′-deoxyadenosine, 1,N6-etheno-2′-deoxyadenosine, N6-hydroxymethyl-2′-deoxyadenosine, and C8-oxo-2′-deoxyguanosine, from different points and zones of the human stomach were semi quantitatively measured by liquid chromatography/tandem mass spectrometry. The differences in the quantity of these DNA adducts from the lesser and greater curvature, the upper, middle and lower third zones, the anterior and posterior wall of the stomach, and the mucosae distant from and near the tumor were compared to determine whether the location preference of cancer in the stomach could be explained by the distribution of these DNA adducts. Comparisons were conducted considering the tumor locations and operation methods. Conclusions: Regarding the DNA adducts investigated, significant differences in quantities and locations in the whole stomach were not noted; thus, these DNA adducts do not explain the preferential occurrence of cancer in particular locations of the human stomach.
Full article
►▼
Show Figures
Open AccessArticle
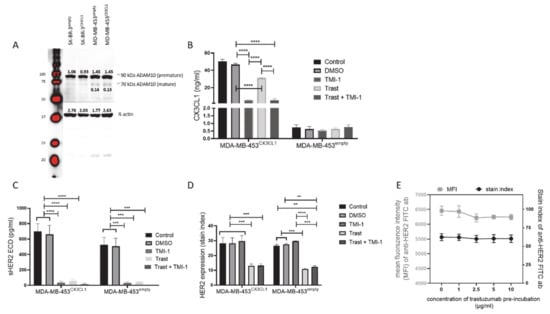
CX3CL1 Overexpression Prevents the Formation of Lung Metastases in Trastuzumab-Treated MDA-MB-453-Based Humanized Tumor Mice (HTM)
by
Anja Kathrin Wege, Tobias F. Dreyer, Attila Teoman, Olaf Ortmann, Gero Brockhoff and Holger Bronger
Cited by 7 | Viewed by 3161
Abstract
CX3CL1 is a multifunctional chemokine that is involved in numerous biological processes, such as immune cell attraction and enhanced tumor immune cell interaction, but also in enhancing tumor cell proliferation and metastasis. The multifarious activity is partially determined by two CX3CL1 isoforms, a
[...] Read more.
CX3CL1 is a multifunctional chemokine that is involved in numerous biological processes, such as immune cell attraction and enhanced tumor immune cell interaction, but also in enhancing tumor cell proliferation and metastasis. The multifarious activity is partially determined by two CX3CL1 isoforms, a membrane-bound and a soluble version generated by proteolytic cleavage through proteases. Here, we investigated the impact of CX3CL1 overexpression in MDA-MB-453 and SK-BR-3 breast cancer cells. Moreover, we evaluated the therapeutic capacity of Matrix-Metalloproteinases-inhibitors TMI-1 and GI254023X in combination with the anti-HER2 antibody trastuzumab in vitro and in vivo. TMI-1 and GI254023X caused a reduced shedding of CX3CL1 and of HER2 in vitro but without effects on tumor cell proliferation or viability. In addition, trastuzumab treatment did not retard MDA-MB-453 cell expansion in vitro unless CX3CL1 was overexpressed upon transfection (MDA-MB-453
CX3CL1). In humanized tumor mice, which show a coexistence of human tumor and human immune system, CX3CL1 overexpression resulted in a slightly enhanced tumor growth. However, trastuzumab treatment attenuated tumor growth of both MDA-MB-453
CX3CL1 and empty vector transfected MDA-MB-453 transplanted mice but showed enhanced efficiency especially in preventing lung metastases in CX3CL1 overexpressing cancer cells. However, TMI-1 did not further enhance the trastuzumab treatment efficacy.
Full article
►▼
Show Figures
Open AccessReview
Recent Advances in Implantation-Based Genetic Modeling of Biliary Carcinogenesis in Mice
by
Masashi Izumiya, Shingo Kato and Yoshitaka Hippo
Cited by 6 | Viewed by 3584
Abstract
Epithelial cells in the biliary system can develop refractory types of cancers, which are often associated with inflammation caused by viruses, parasites, stones, and chemicals. Genomic studies have revealed recurrent genetic changes and deregulated signaling pathways in biliary tract cancer (BTC). The causal
[...] Read more.
Epithelial cells in the biliary system can develop refractory types of cancers, which are often associated with inflammation caused by viruses, parasites, stones, and chemicals. Genomic studies have revealed recurrent genetic changes and deregulated signaling pathways in biliary tract cancer (BTC). The causal roles have been at least partly clarified using various genetically engineered mice. Technical advances in Cre-LoxP technology, together with hydrodynamic tail injection, CRISPR/Cas9 technology, in vivo electroporation, and organoid culture have enabled more precise modeling of BTC. Organoid-based genetic modeling, combined with implantation in mice, has recently drawn attention as a means to accelerate the development of BTC models. Although each model may not perfectly mimic the disease, they can complement one another, or two different approaches can be integrated to establish a novel model. In addition, a comparison of the outcomes among these models with the same genotype provides mechanistic insights into the interplay between genetic alterations and the microenvironment in the pathogenesis of BTCs. Here, we review the current status of genetic models of BTCs in mice to provide information that facilitates the wise selection of models and to inform the future development of ideal disease models.
Full article
►▼
Show Figures
Open AccessReview
Mouse Models for Deciphering the Impact of Homologous Recombination on Tumorigenesis
by
Gabriel Matos-Rodrigues, Emmanuelle Martini and Bernard S. Lopez
Cited by 6 | Viewed by 3624
Abstract
Homologous recombination (HR) is a fundamental evolutionarily conserved process that plays prime role(s) in genome stability maintenance through DNA repair and through the protection and resumption of arrested replication forks. Many HR genes are deregulated in cancer cells. Notably, the breast cancer genes
[...] Read more.
Homologous recombination (HR) is a fundamental evolutionarily conserved process that plays prime role(s) in genome stability maintenance through DNA repair and through the protection and resumption of arrested replication forks. Many HR genes are deregulated in cancer cells. Notably, the breast cancer genes
BRCA1 and
BRCA2, two important HR players, are the most frequently mutated genes in familial breast and ovarian cancer. Transgenic mice constitute powerful tools to unravel the intricate mechanisms controlling tumorigenesis in vivo. However, the genes central to HR are essential in mammals, and their knockout leads to early embryonic lethality in mice. Elaborated strategies have been developed to overcome this difficulty, enabling one to analyze the consequences of HR disruption in vivo. In this review, we first briefly present the molecular mechanisms of HR in mammalian cells to introduce each factor in the HR process. Then, we present the different mouse models of HR invalidation and the consequences of HR inactivation on tumorigenesis. Finally, we discuss the use of mouse models for the development of targeted cancer therapies as well as perspectives on the future potential for understanding the mechanisms of HR inactivation-driven tumorigenesis in vivo.
Full article
►▼
Show Figures
Open AccessArticle
Colibactin-Producing Escherichia coli Induce the Formation of Invasive Carcinomas in a Chronic Inflammation-Associated Mouse Model
by
Laurène Salesse, Cécily Lucas, My Hanh Thi Hoang, Pierre Sauvanet, Alexandra Rezard, Philip Rosenstiel, Christelle Damon-Soubeyrand, Nicolas Barnich, Catherine Godfraind, Guillaume Dalmasso and Hang Thi Thu Nguyen
Cited by 24 | Viewed by 3308
Abstract
Background:
Escherichia coli producing the genotoxin colibactin (CoPEC or colibactin-producing
E. coli) abnormally colonize the colonic mucosa of colorectal cancer (CRC) patients. We previously showed that deficiency of autophagy in intestinal epithelial cells (IECs) enhances CoPEC-induced colorectal carcinogenesis in
ApcMin/+ mice.
[...] Read more.
Background:
Escherichia coli producing the genotoxin colibactin (CoPEC or colibactin-producing
E. coli) abnormally colonize the colonic mucosa of colorectal cancer (CRC) patients. We previously showed that deficiency of autophagy in intestinal epithelial cells (IECs) enhances CoPEC-induced colorectal carcinogenesis in
ApcMin/+ mice. Here, we tested if CoPEC trigger tumorigenesis in a mouse model lacking genetic susceptibility or the use of carcinogen. Methods: Mice with autophagy deficiency in IECs (
Atg16l1∆IEC) or wild-type mice (
Atg16l1flox/flox) were infected with the CoPEC 11G5 strain or the mutant 11G5∆clbQ incapable of producing colibactin and subjected to 12 cycles of DSS treatment to induce chronic colitis. Mouse colons were used for histological assessment, immunohistochemical and immunoblot analyses for DNA damage marker.
Results: 11G5 or 11G5∆clbQ infection increased clinical and histological inflammation scores, and these were further enhanced by IEC-specific autophagy deficiency. 11G5 infection, but not 11G5∆clbQ infection, triggered the formation of invasive carcinomas, and this was further increased by autophagy deficiency. The increase in invasive carcinomas was correlated with enhanced DNA damage and independent of inflammation.
Conclusions: CoPEC induce colorectal carcinogenesis in a CRC mouse model lacking genetic susceptibility and carcinogen. This work highlights the role of (i) CoPEC as a driver of CRC development, and (ii) autophagy in inhibiting the carcinogenic properties of CoPEC.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
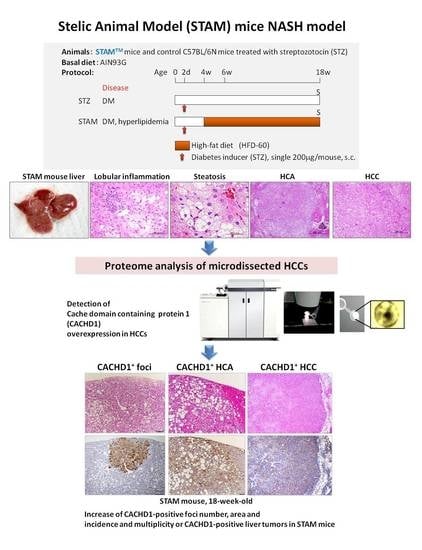
Cache Domain Containing 1 Is a Novel Marker of Non-Alcoholic Steatohepatitis-Associated Hepatocarcinogenesis
by
Anna Kakehashi, Arpamas Chariyakornkul, Shugo Suzuki, Napaporn Khuanphram, Kumiko Tatsumi, Shotaro Yamano, Masaki Fujioka, Min Gi, Rawiwan Wongpoomchai and Hideki Wanibuchi
Cited by 5 | Viewed by 3427
Abstract
In the present study, potential molecular biomarkers of NASH hepatocarcinogenesis were investigated using the STAM mice NASH model, characterized by impaired insulin secretion and development of insulin resistance. In this model, 2-days-old C57BL/6N mice were subjected to a single subcutaneous (s.c.) injection of
[...] Read more.
In the present study, potential molecular biomarkers of NASH hepatocarcinogenesis were investigated using the STAM mice NASH model, characterized by impaired insulin secretion and development of insulin resistance. In this model, 2-days-old C57BL/6N mice were subjected to a single subcutaneous (s.c.) injection of 200 μg streptozotocin (STZ) to induce diabetes mellitus (DM). Four weeks later, mice were administered high-fat diet (HFD) HFD-60 for 14 weeks (STAM group), or fed control diet (STZ group). Eighteen-week-old mice were euthanized to allow macroscopic, microscopic, histopathological, immunohistochemical and proteome analyses. The administration of HFD to STZ-treated mice induced significant fat accumulation and fibrosis development in the liver, which progressed to NASH, and rise of hepatocellular adenomas (HCAs) and carcinomas (HCCs). In 18-week-old animals, a significant increase in the incidence and multiplicity of HCAs and HCCs was found. On the basis of results of proteome analysis of STAM mice HCCs, a novel highly elevated protein in HCCs, cache domain-containing 1 (CACHD1), was chosen as a potential NASH-HCC biomarker candidate. Immunohistochemical assessment demonstrated that STAM mice liver basophilic, eosinophilic and mixed-type altered foci, HCAs and HCCs were strongly positive for CACHD1. The number and area of CACHD1-positive foci, and cell proliferation index in the area of foci in mice of the STAM group were significantly increased compared to that of STZ group. In vitro siRNA knockdown of CACHD1 in human Huh7 and HepG2 liver cancer cell lines resulted in significant inhibition of cell survival and proliferation. Analysis of the proteome of knockdown cells indicated that apoptosis and autophagy processes could be activated. From these results, CACHD1 is an early NASH-associated biomarker of liver preneoplastic and neoplastic lesions, and a potential target protein in DM/NASH-associated hepatocarcinogenesis.
Full article
►▼
Show Figures
Open AccessArticle
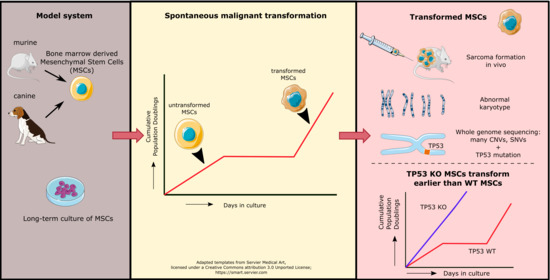
Transformed Canine and Murine Mesenchymal Stem Cells as a Model for Sarcoma with Complex Genomics
by
Natasja Franceschini, Bas Verbruggen, Marianna A. Tryfonidou, Alwine B. Kruisselbrink, Hans Baelde, Karin E. de Visser, Karoly Szuhai, Anne-Marie Cleton-Jansen and Judith V. M. G. Bovée
Cited by 7 | Viewed by 2741
Abstract
Sarcomas are rare mesenchymal tumors with a broad histological spectrum, but they can be divided into two groups based on molecular pathology: sarcomas with simple or complex genomics. Tumors with complex genomics can have aneuploidy and copy number gains and losses, which hampers
[...] Read more.
Sarcomas are rare mesenchymal tumors with a broad histological spectrum, but they can be divided into two groups based on molecular pathology: sarcomas with simple or complex genomics. Tumors with complex genomics can have aneuploidy and copy number gains and losses, which hampers the detection of early, initiating events in tumorigenesis. Often, no benign precursors are known, which is why good models are essential. The mesenchymal stem cell (MSC) is the presumed cell of origin of sarcoma. In this study, MSCs of murine and canine origin are used as a model to identify driver events for sarcomas with complex genomic alterations as they transform spontaneously after long-term culture. All transformed murine but not canine MSCs formed sarcomas after subcutaneous injection in mice. Using whole genome sequencing, spontaneously transformed murine and canine MSCs displayed a complex karyotype with aneuploidy, point mutations, structural variants, inter-chromosomal translocations, and copy number gains and losses. Cross-species analysis revealed that point mutations in
Tp53/Trp53 are common in transformed murine and canine MSCs. Murine MSCs with a cre-recombinase induced deletion of exon 2–10 of
Trp53 transformed earlier compared to wild-type murine MSCs, confirming the contribution of loss of p53 to spontaneous transformation. Our comparative approach using transformed murine and canine MSCs points to a crucial role for p53 loss in the formation of sarcomas with complex genomics.
Full article
►▼
Show Figures
Open AccessArticle
The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis
by
Kelly Harper, Anna Yatsyna, Martine Charbonneau, Karine Brochu-Gaudreau, Alexis Perreault, Claudio Jeldres, Patrick P. McDonald and Claire M. Dubois
Cited by 22 | Viewed by 3459
Abstract
Hypoxia in the tumor microenvironment is a negative prognostic factor associated with tumor progression and metastasis, and therefore represents an attractive therapeutic target for anti-tumor therapy. To test the effectiveness of novel hypoxia-targeting drugs, appropriate preclinical models that recreate tumor hypoxia are essential.
[...] Read more.
Hypoxia in the tumor microenvironment is a negative prognostic factor associated with tumor progression and metastasis, and therefore represents an attractive therapeutic target for anti-tumor therapy. To test the effectiveness of novel hypoxia-targeting drugs, appropriate preclinical models that recreate tumor hypoxia are essential. The chicken ChorioAllantoic Membrane (CAM) assay is increasingly used as a rapid cost-effective in vivo drug-testing platform that recapitulates many aspects of human cancers. However, it remains to be determined whether this model recreates the hypoxic microenvironment of solid tumors. To detect hypoxia in the CAM model, the hypoxic marker pimonidazole was injected into the vasculature of tumor-bearing CAM, and hypoxia-dependent gene expression was analyzed. We observed that the CAM model effectively supports the development of hypoxic zones in a variety of human tumor cell line-derived and patient’s tumor fragment-derived xenografts. The treatment of both patient and cell line-derived CAM xenografts with modulators of angiogenesis significantly altered the formation of hypoxic zones within the xenografts. Furthermore, the changes in hypoxia translated into modulated levels of chick liver metastasis as measured by Alu-based assay. These findings demonstrate that the CAM xenograft model is a valuable in vivo platform for studying hypoxia that could facilitate the identification and testing of drugs targeting this tumor microenvironment.
Full article
►▼
Show Figures
Open AccessReview
The Japanese Wild-Derived Inbred Mouse Strain, MSM/Ms in Cancer Research
by
Kazuhiro Okumura, Megumi Saito, Eriko Isogai and Yuichi Wakabayashi
Cited by 7 | Viewed by 3195
Abstract
MSM/Ms is a unique inbred mouse strain derived from the Japanese wild mouse,
Mus musculus molossinus, which has been approximately 1 million years genetically distant from standard inbred mouse strains mainly derived from
M. m. domesticus. Due to its genetic divergence,
[...] Read more.
MSM/Ms is a unique inbred mouse strain derived from the Japanese wild mouse,
Mus musculus molossinus, which has been approximately 1 million years genetically distant from standard inbred mouse strains mainly derived from
M. m. domesticus. Due to its genetic divergence, MSM/Ms has been broadly used in linkage studies. A bacterial artificial chromosome (BAC) library was constructed for the MSM/Ms genome, and sequence analysis of the MSM/Ms genome showed approximately 1% of nucleotides differed from those in the commonly used inbred mouse strain, C57BL/6J. Therefore, MSM/Ms mice are thought to be useful for functional genome studies. MSM/Ms mice show unique characteristics of phenotypes, including its smaller body size, resistance to high-fat-diet-induced diabetes, high locomotive activity, and resistance to age-onset hearing loss, inflammation, and tumorigenesis, which are distinct from those of common inbred mouse strains. Furthermore, ES (Embryonic Stem) cell lines established from MSM/Ms allow the MSM/Ms genome to be genetically manipulated. Therefore, genomic and phenotypic analyses of MSM/Ms reveal novel insights into gene functions that were previously not obtained from research on common laboratory strains. Tumorigenesis-related MSM/Ms-specific genetic traits have been intensively investigated in Japan. Furthermore, radiation-induced thymic lymphomas and chemically-induced skin tumors have been extensively examined using MSM/Ms.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Inflammation-Related Carcinogenesis: Lessons from Animal Models to Clinical Aspects
by
Futoshi Okada, Runa Izutsu, Keisuke Goto and Mitsuhiko Osaki
Cited by 14 | Viewed by 3825
Abstract
Inflammation-related carcinogenesis has long been known as one of the carcinogenesis patterns in humans. Common carcinogenic factors are inflammation caused by infection with pathogens or the uptake of foreign substances from the environment into the body. Inflammation-related carcinogenesis as a cause for cancer-related
[...] Read more.
Inflammation-related carcinogenesis has long been known as one of the carcinogenesis patterns in humans. Common carcinogenic factors are inflammation caused by infection with pathogens or the uptake of foreign substances from the environment into the body. Inflammation-related carcinogenesis as a cause for cancer-related death worldwide accounts for approximately 20%, and the incidence varies widely by continent, country, and even region of the country and can be affected by economic status or development. Many novel approaches are currently available concerning the development of animal models to elucidate inflammation-related carcinogenesis. By learning from the oldest to the latest animal models for each organ, we sought to uncover the essential common causes of inflammation-related carcinogenesis. This review confirmed that a common etiology of organ-specific animal models that mimic human inflammation-related carcinogenesis is prolonged exudation of inflammatory cells. Genotoxicity or epigenetic modifications by inflammatory cells resulted in gene mutations or altered gene expression, respectively. Inflammatory cytokines/growth factors released from inflammatory cells promote cell proliferation and repair tissue injury, and inflammation serves as a “carcinogenic niche”, because these fundamental biological events are common to all types of carcinogenesis, not just inflammation-related carcinogenesis. Since clinical strategies are needed to prevent carcinogenesis, we propose the therapeutic apheresis of inflammatory cells as a means of eliminating fundamental cause of inflammation-related carcinogenesis.
Full article
►▼
Show Figures
Open AccessReview
Carcinogenesis as Side Effects of Iron and Oxygen Utilization: From the Unveiled Truth toward Ultimate Bioengineering
by
Shinya Toyokuni, Yingyi Kong, Zhen Cheng, Kotaro Sato, Shotaro Hayashi, Fumiya Ito, Li Jiang, Izumi Yanatori, Yasumasa Okazaki and Shinya Akatsuka
Cited by 27 | Viewed by 3620
Abstract
Evolution from the first life on earth to humans took ~3.8 billion years. During the time there have been countless struggles among the species. Mycobacterium tuberculosis was the last major uncontrollable species against the human public health worldwide. After the victory with antibiotics,
[...] Read more.
Evolution from the first life on earth to humans took ~3.8 billion years. During the time there have been countless struggles among the species. Mycobacterium tuberculosis was the last major uncontrollable species against the human public health worldwide. After the victory with antibiotics, cancer has become the leading cause of death since 1981 in Japan. Considering that life inevitably depends on ceaseless electron transfers through iron and oxygen, we believe that carcinogenesis is intrinsically unavoidable side effects of using iron and oxygen. Many animal models unequivocally revealed that excess iron is a risk for carcinogenesis. This is supported by a variety of human epidemiological data on cancer risk and prognosis. Cancer is basically a disease of the genome with persistently activated oncogenes and inactivated tumor suppressor genes through which iron addiction with ferroptosis-resistance is maintained. Engineering has made a great advance in the past 50 years. In particular, nanotechnology is distinct in that the size of the engineered molecules is similar to that of our biomolecules. While some nano-molecules are found carcinogenic, there are principles to avoid such carcinogenicity with a smart possibility to use nano-molecules to specifically kill cancer cells. Non-thermal plasma is another modality to fight against cancer.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
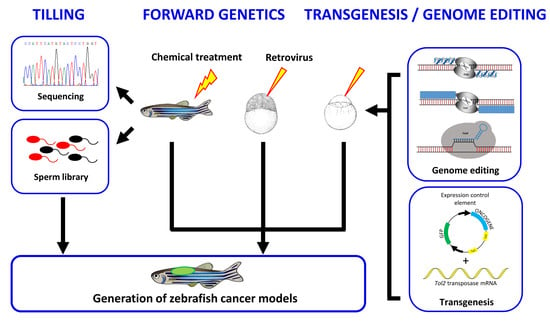
Genetic Engineering of Zebrafish in Cancer Research
by
Ludivine Raby, Pamela Völkel, Xuefen Le Bourhis and Pierre-Olivier Angrand
Cited by 28 | Viewed by 7612
Abstract
Zebrafish (
Danio rerio) is an excellent model to study a wide diversity of human cancers. In this review, we provide an overview of the genetic and reverse genetic toolbox allowing the generation of zebrafish lines that develop tumors. The large spectrum
[...] Read more.
Zebrafish (
Danio rerio) is an excellent model to study a wide diversity of human cancers. In this review, we provide an overview of the genetic and reverse genetic toolbox allowing the generation of zebrafish lines that develop tumors. The large spectrum of genetic tools enables the engineering of zebrafish lines harboring precise genetic alterations found in human patients, the generation of zebrafish carrying somatic or germline inheritable mutations or zebrafish showing conditional expression of the oncogenic mutations. Comparative transcriptomics demonstrate that many of the zebrafish tumors share molecular signatures similar to those found in human cancers. Thus, zebrafish cancer models provide a unique in vivo platform to investigate cancer initiation and progression at the molecular and cellular levels, to identify novel genes involved in tumorigenesis as well as to contemplate new therapeutic strategies.
Full article
►▼
Show Figures
Open AccessArticle
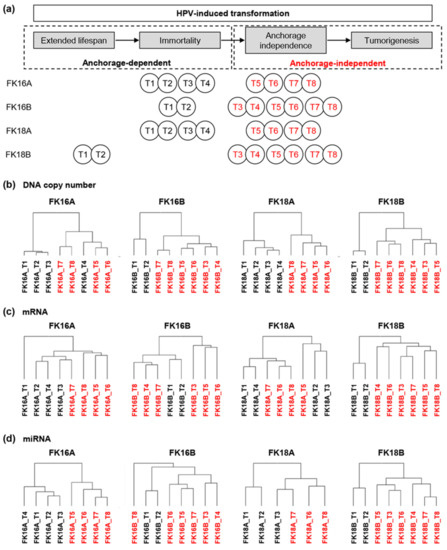
Identification of Deregulated Pathways, Key Regulators, and Novel miRNA-mRNA Interactions in HPV-Mediated Transformation
by
Iris Babion, Viktorian Miok, Annelieke Jaspers, Angelina Huseinovic, Renske D. M. Steenbergen, Wessel N. van Wieringen and Saskia M. Wilting
Cited by 25 | Viewed by 4421
Abstract
Next to a persistent infection with high-risk human papillomavirus (HPV), molecular changes are required for the development of cervical cancer. To identify which molecular alterations drive carcinogenesis, we performed a comprehensive and longitudinal molecular characterization of HPV-transformed keratinocyte cell lines. Comparative genomic hybridization,
[...] Read more.
Next to a persistent infection with high-risk human papillomavirus (HPV), molecular changes are required for the development of cervical cancer. To identify which molecular alterations drive carcinogenesis, we performed a comprehensive and longitudinal molecular characterization of HPV-transformed keratinocyte cell lines. Comparative genomic hybridization, mRNA, and miRNA expression analysis of four HPV-containing keratinocyte cell lines at eight different time points was performed. Data was analyzed using unsupervised hierarchical clustering, integrated longitudinal expression analysis, and pathway enrichment analysis. Biological relevance of identified key regulatory genes was evaluated in vitro and dual-luciferase assays were used to confirm predicted miRNA-mRNA interactions. We show that the acquisition of anchorage independence of HPV-containing keratinocyte cell lines is particularly associated with copy number alterations. Approximately one third of differentially expressed mRNAs and miRNAs was directly attributable to copy number alterations. Focal adhesion, TGF-beta signaling, and mTOR signaling pathways were enriched among these genes. PITX2 was identified as key regulator of TGF-beta signaling and inhibited cell growth in vitro, most likely by inducing cell cycle arrest and apoptosis. Predicted miRNA-mRNA interactions miR-221-3p_BRWD3, miR-221-3p_FOS, and miR-138-5p_PLXNB2 were confirmed in vitro. Integrated longitudinal analysis of our HPV-induced carcinogenesis model pinpointed relevant interconnected molecular changes and crucial signaling pathways in HPV-mediated transformation.
Full article
►▼
Show Figures
Open AccessArticle
Cooperation between SS18-SSX1 and miR-214 in Synovial Sarcoma Development and Progression
by
Miwa Tanaka, Mizuki Homme, Yukari Yamazaki, Keisuke Ae, Seiichi Matsumoto, Subbaya Subramanian and Takuro Nakamura
Cited by 13 | Viewed by 4256
Abstract
SS18-SSX fusion proteins play a central role in synovial sarcoma development, although, the genetic network and mechanisms of synovial sarcomagenesis remain unknown. We established a new ex vivo synovial sarcoma mouse model through retroviral-mediated gene transfer of
SS18-SSX1 into mouse embryonic mesenchymal cells
[...] Read more.
SS18-SSX fusion proteins play a central role in synovial sarcoma development, although, the genetic network and mechanisms of synovial sarcomagenesis remain unknown. We established a new ex vivo synovial sarcoma mouse model through retroviral-mediated gene transfer of
SS18-SSX1 into mouse embryonic mesenchymal cells followed by subcutaneous transplantation into nude mice. This approach successfully induced subcutaneous tumors in 100% recipients, showing invasive proliferation of short spindle tumor cells with occasional biphasic appearance. Cytokeratin expression was observed in epithelial components in tumors and expression of TLE1 and BCL2 was also shown. Gene expression profiling indicated SWI/SNF pathway modulation by
SS18-SSX1 introduction into mesenchymal cells and
Tle1 and
Atf2 upregulation in tumors. These findings indicate that the model exhibits phenotypes typical of human synovial sarcoma. Retroviral tagging of the tumor identified 15 common retroviral integration sites within the
Dnm3 locus as the most frequent in 30 mouse synovial sarcomas.
miR-199a2 and
miR-214 upregulation within the
Dnm3 locus was observed.
SS18-SSX1 and
miR-214 cointroduction accelerated sarcoma onset, indicating that
miR-214 is a cooperative oncomiR in synovial sarcomagenesis.
miR-214 functions in a cell non-autonomous manner, promoting cytokine gene expression (e.g.,
Cxcl15/IL8). Our results emphasize the role of
miR-214 in tumor development and disease progression.
Full article
►▼
Show Figures
Open AccessArticle
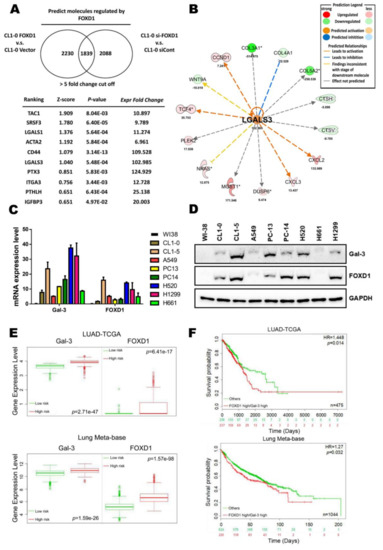
FOXD1 and Gal-3 Form a Positive Regulatory Loop to Regulate Lung Cancer Aggressiveness
by
Chien-Hsiu Li, Yu-Chan Chang, Michael Hsiao and Shu-Mei Liang
Cited by 27 | Viewed by 4365
Abstract
Dysregulation of forkhead box D1 (FOXD1) is known to promote tumor progression; however, its molecular mechanism of action is unclear. Based on microarray analysis, we identified galectin-3/LGALS3 (Gal-3) as a potential downstream target of FOXD1, as FOXD1 transactivated
Gal-3 by interacting with the
[...] Read more.
Dysregulation of forkhead box D1 (FOXD1) is known to promote tumor progression; however, its molecular mechanism of action is unclear. Based on microarray analysis, we identified galectin-3/LGALS3 (Gal-3) as a potential downstream target of FOXD1, as FOXD1 transactivated
Gal-3 by interacting with the
Gal-3 promoter to upregulate Gal-3 in FOXD1-overexpressing CL1-0 lung cancer cells. Ectopic expression of FOXD1 increased the expression of Gal-3 and the growth and motility of lung cancer cells, whereas depletion of Gal-3 attenuated FOXD1-mediated tumorigenesis. ERK1/2 interacted with FOXD1 in the cytosol and translocated FOXD1 into the nucleus to activate
Gal-3. Gal-3 in turn upregulated FOXD1 via the transcription factor proto-oncogene 1 (ETS-1) to transactivate
FOXD1. The increase in ETS-1/FOXD1 expression by Gal-3 was through Gal-3-mediated integrin-β1 (ITGβ1) signaling. The overexpression of both FOXD1 and Gal-3 form a positive regulatory loop to promote lung cancer aggressiveness. Moreover, both FOXD1 and Gal-3 were positively correlated in human lung cancer tissues. Our findings demonstrated that FOXD1 and Gal-3 form a positive feedback loop in lung cancer, and interference of this loop may serve as an effective therapeutic target for the treatment of lung cancers, particularly those related to dysregulation of Gal-3.
Full article
►▼
Show Figures