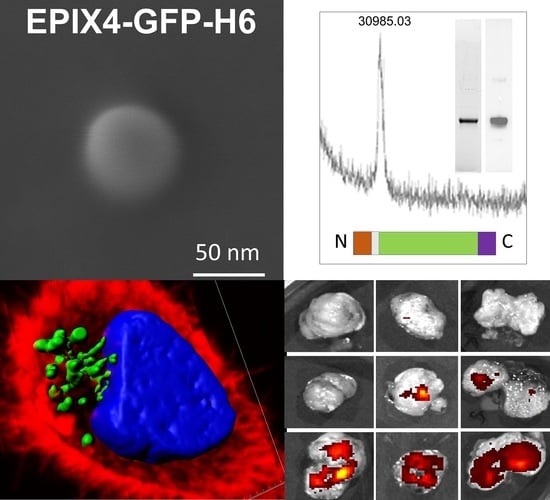
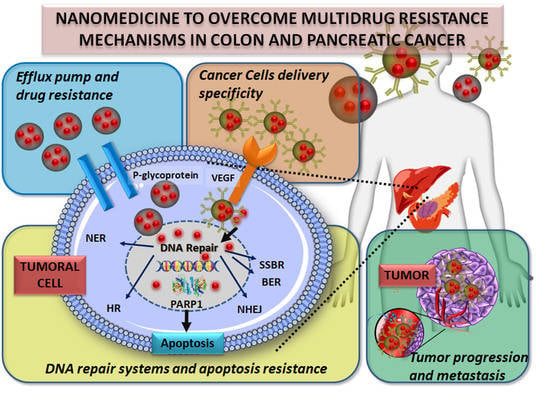
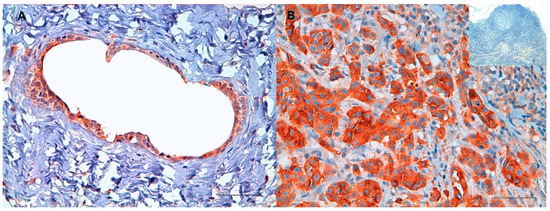
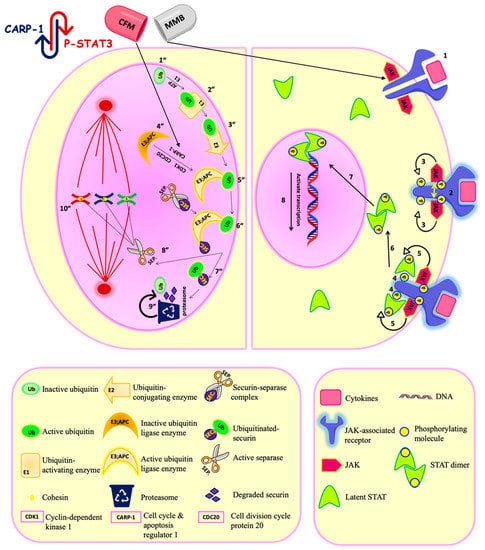
Cancer Nanomedicine
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 133213Editor
Interests: nanomedicine; cancer nanotechnology; targeted drug delivery; pancreatic cancer; theranostics
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
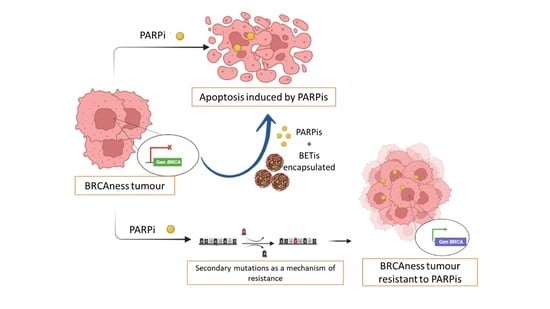
Cancer treatments are often hindered by the lack of drug specificity, poor physicochemical properties of active pharmaceutical ingredients, poor penetration ability and drug resistance. With the discovery and characterization of an increasing number of cancer types with little improvement of the ability to diagnose, treatment options or patient prognosis, more advanced technologies are urgently required.
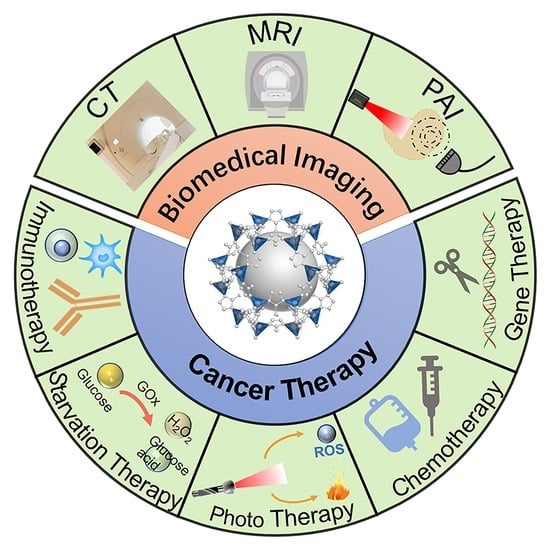
Nanotechnology defines particulates within the 1x10-9 m range. Particulates within the nano-sized domain often exhibit unique properties compared to their larger size scale. These can be exploited in biomedicine for applications such as imaging, cell sorting, drug delivery and targeting.
Cancer nanomedicine is rapidly becoming one of the leading areas of promise for cancer therapy, with first-generation treatments already available to patients. This Collection invites articles and reviews which encompass the many areas under the nanomedicine umbrella, including diagnostics, drug delivery and advanced therapies.
Dr. Clare Hoskins
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Nanomedicine
- targeted therapy
- image guidance
- tumour penetration
- stimuli-responsive
- theranostics
- drug delivery
- nanoparticle
- nanoencapsulation
- diagnosis