Applications of Stem Cells in Cardiovascular Functional Genomics 2022
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Stem Cells".
Viewed by 9542
Share This Topical Collection
Editor
 Prof. Dr. Agapios Sachinidis
Prof. Dr. Agapios Sachinidis
 Prof. Dr. Agapios Sachinidis
Prof. Dr. Agapios Sachinidis
E-Mail
Website
Collection Editor
Institute of Neurophysiology and Center for Molecular Medicine Cologne (CMMC), University of Cologne, Robert-Koch-Str. 39, 50931 Cologne, Germany
Interests: molecular genetics and genomics of stem cells; stem cell cardiovascular genomics; developmental/differentiation; toxicity gene signatures and pathways
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
The regular development and maintenance of an intact cardiovascular system involve fine-tuned complex gene expression pathways which coordinate the development and function of the cardiovascular system. The field of cardiovascular functional genomics aims to identify regular genes and signal transduction pathways to obtain a better understanding of the development and progress of cardiovascular diseases (CVDs). Because of the strict ethical aspects, to date, functional genomics studies of the cardiovascular system have been executed using small animal studies. However, not all animal functional genomics studies can be transferred to humans. Moreover, even though animal studies have significantly contributed to the functional genomics of the cardiovascular system (CVS), these studies are very costly and time-consuming and cannot be executed in a high-throughput approach.
Pluripotent stem cells (PSCs) including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have been shown to partly recapitulate embryonic development in vivo. Moreover, human cardiomyocytes from PSCs in combination with advanced genomics and live-cell imaging techniques are applied for discovering genomic networks of functional relevance, involved in the development of heart diseases. It is hoped that progress in this field will contribute to personalized medicine for developing better and novel therapeutic tools for the treatment of heart diseases. The present topic is focused on the latest progress in functional genomics (including epigenomics) of the heart system based on the PSC model. Emphasis will be placed on the question of how this emerging field will contribute to the discovery of novel mechanisms, pathways, and drugs which are relevant to therapeutic applications of heart diseases. In addition to original manuscripts, review manuscripts discussing limitations and advances of this field will also be considered for publication.
Prof. Dr. Agapios Sachinidis
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- pluripotent stem cells
- functional genomic and epigenomics
- signal transduction pathways
- cardiovascular diseases
- cardiovascular toxicity
Published Papers (3 papers)
Open AccessArticle
Reprogramming Megakaryocytes for Controlled Release of Platelet-like Particles Carrying a Single-Chain Thromboxane A2 Receptor-G-Protein Complex with Therapeutic Potential
by
Renzhong Lu, Yan Li, Anna Xu, Bridgette King and Ke-He Ruan
Viewed by 1425
Abstract
In this study, we reported that novel single-chain fusion proteins linking thromboxane A
2 (TXA
2) receptor (TP) to a selected G-protein α-subunit q (SC-TP-Gαq) or to α-subunit s (SC-TP-Gαs) could be stably expressed in megakaryocytes (MKs). We tested the MK-released platelet-linked
[...] Read more.
In this study, we reported that novel single-chain fusion proteins linking thromboxane A
2 (TXA
2) receptor (TP) to a selected G-protein α-subunit q (SC-TP-Gαq) or to α-subunit s (SC-TP-Gαs) could be stably expressed in megakaryocytes (MKs). We tested the MK-released platelet-linked particles (PLPs) to be used as a vehicle to deliver the overexpressed SC-TP-Gαq or the SC-TP-Gαs to regulate human platelet function. To understand how the single-chain TP-Gα fusion proteins could regulate opposite platelet activities by an identical ligand TXA
2, we tested their dual functions—binding to ligands and directly linking to different signaling pathways within a single polypeptide chain—using a 3D structural model. The immature MKs were cultured and transfected with cDNAs constructed from structural models of the individual SC-TP-Gαq and SC-TP-Gαs, respectively. After transient expression was identified, the immature MKs stably expressing SC-TP-Gαq or SC-TP-Gαs (stable cell lines) were selected. The stable cell lines were induced into mature MKs which released PLPs. Western blot analysis confirmed that the released PLPs were carrying the recombinant SC-TP-Gαq or SC-TP-Gαs. Flow cytometry analysis showed that the PLPs carrying SC-TP-Gαq were able to perform the activity by promoting platelet aggregation. In contrast, PLPs carrying SC-TP-Gαs reversed Gq to Gs signaling to inhibit platelet aggregation. This is the first time demonstrating that SC-TP-Gαq and SC-TP-Gαs were successfully overexpressed in MK cells and released as PLPs with proper folding and programmed biological activities. This bio-engineering led to the formation of two sets of biologically active PLP forms mediating calcium and cAMP signaling, respectively. As a result, these PLPs are able to bind to identical endogenous TXA
2 with opposite activities, inhibiting and promoting platelet aggregation as reprogrammed for therapeutic process. Results also demonstrated that the nucleus-free PLPs could be used to deliver recombinant membrane-bound GPCRs to regulate cellular activity in general.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
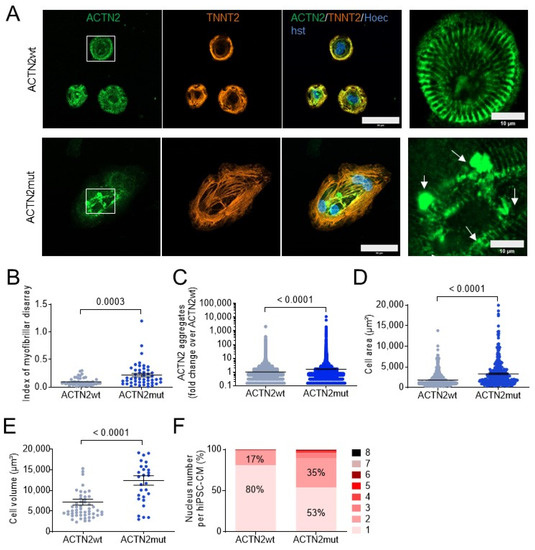
ACTN2 Mutant Causes Proteopathy in Human iPSC-Derived Cardiomyocytes
by
Antonia T. L. Zech, Maksymilian Prondzynski, Sonia R. Singh, Niels Pietsch, Ellen Orthey, Erda Alizoti, Josefine Busch, Alexandra Madsen, Charlotta S. Behrens, Moritz Meyer-Jens, Giulia Mearini, Marc D. Lemoine, Elisabeth Krämer, Diogo Mosqueira, Sanamjeet Virdi, Daniela Indenbirken, Maren Depke, Manuela Gesell Salazar, Uwe Völker, Ingke Braren, William T. Pu, Thomas Eschenhagen, Elke Hammer, Saskia Schlossarek and Lucie Carrieradd
Show full author list
remove
Hide full author list
| Viewed by 3852
Abstract
Genetic variants in α-actinin-2 (ACTN2) are associated with several forms of (cardio)myopathy. We previously reported a heterozygous missense (c.740C>T)
ACTN2 gene variant, associated with hypertrophic cardiomyopathy, and characterized by an electro-mechanical phenotype in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). Here, we created
[...] Read more.
Genetic variants in α-actinin-2 (ACTN2) are associated with several forms of (cardio)myopathy. We previously reported a heterozygous missense (c.740C>T)
ACTN2 gene variant, associated with hypertrophic cardiomyopathy, and characterized by an electro-mechanical phenotype in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs). Here, we created with CRISPR/Cas9 genetic tools two heterozygous functional knock-out hiPSC lines with a second wild-type (ACTN2wt) and missense ACTN2 (ACTN2mut) allele, respectively. We evaluated their impact on cardiomyocyte structure and function, using a combination of different technologies, including immunofluorescence and live cell imaging, RNA-seq, and mass spectrometry. This study showed that ACTN2mut presents a higher percentage of multinucleation, protein aggregation, hypertrophy, myofibrillar disarray, and activation of both the ubiquitin-proteasome system and the autophagy-lysosomal pathway as compared to ACTN2wt in 2D-cultured hiPSC-CMs. Furthermore, the expression of ACTN2mut was associated with a marked reduction of sarcomere-associated protein levels in 2D-cultured hiPSC-CMs and force impairment in engineered heart tissues. In conclusion, our study highlights the activation of proteolytic systems in ACTN2mut hiPSC-CMs likely to cope with ACTN2 aggregation and therefore directs towards proteopathy as an additional cellular pathology caused by this
ACTN2 variant, which may contribute to human
ACTN2-associated cardiomyopathies.
Full article
►▼
Show Figures
Open AccessArticle
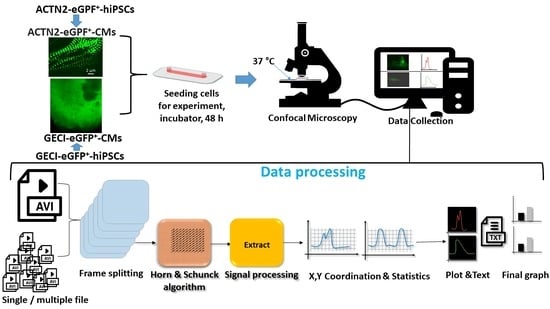
Live-Cell Imaging of the Contractile Velocity and Transient Intracellular Ca2+ Fluctuations in Human Stem Cell-Derived Cardiomyocytes
by
Aviseka Acharya, Harshal Nemade, Krishna Rajendra Prasad, Khadija Khan, Jürgen Hescheler, Nick Blackburn, Ruth Hemmersbach, Symeon Papadopoulos and Agapios Sachinidis
Cited by 7 | Viewed by 3490
Abstract
Live-cell imaging techniques are essential for acquiring vital physiological and pathophysiological knowledge to understand and treat heart disease. For live-cell imaging of transient alterations of [Ca
2+]
i in human cardiomyocytes, we engineered human-induced pluripotent stem cells carrying a genetically-encoded Ca
2+
[...] Read more.
Live-cell imaging techniques are essential for acquiring vital physiological and pathophysiological knowledge to understand and treat heart disease. For live-cell imaging of transient alterations of [Ca
2+]
i in human cardiomyocytes, we engineered human-induced pluripotent stem cells carrying a genetically-encoded Ca
2+-indicator (GECI). To monitor sarcomere shortening and relaxation in cardiomyocytes in real-time, we generated a α-cardiac actinin (ACTN2)-copepod (cop) green fluorescent protein (GFP
+)-human-induced pluripotent stem cell line by using the CRISPR-Cas9 and a homology directed recombination approach. The engineered human-induced pluripotent stem cells were differentiated in transgenic GECI-enhanced GFP
+-cardiomyocytes and ACTN2-copGFP
+-cardiomyocytes, allowing real-time imaging of [Ca
2+]
i transients and live recordings of the sarcomere shortening velocity of ACTN2-copGFP
+-cardiomyocytes. We developed a video analysis software tool to quantify various parameters of sarcoplasmic Ca
2+ fluctuations recorded during contraction of cardiomyocytes and to calculate the contraction velocity of cardiomyocytes in the presence and absence of different drugs affecting cardiac function. Our cellular and software tool not only proved the positive and negative inotropic and lusitropic effects of the tested cardioactive drugs but also quantified the expected effects precisely. Our platform will offer a human-relevant in vitro alternative for high-throughput drug screenings, as well as a model to explore the underlying mechanisms of cardiac diseases.
Full article
►▼
Show Figures