Gut Microbiota-Derived Metabolites and Host Gut-Brain Communication
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Immunology".
Viewed by 85440Editors
Interests: microbiota-gut-brain axis; enteric nervous system; gut neuromuscular plasticity; dysbiosis; irritable bowel syndrome (IBS); Inflammatory Bowel Disease (IBD)
Special Issues, Collections and Topics in MDPI journals
Interests: persistent viral infections; microbiota-gut-brain axis; viral pathogenesis; diagnostic of viral infections
Topical Collection Information
Dear Colleagues,
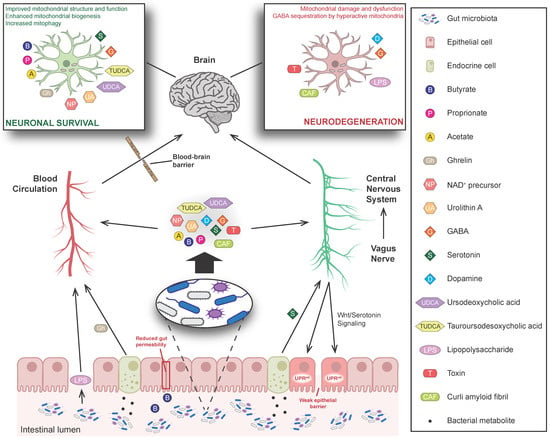
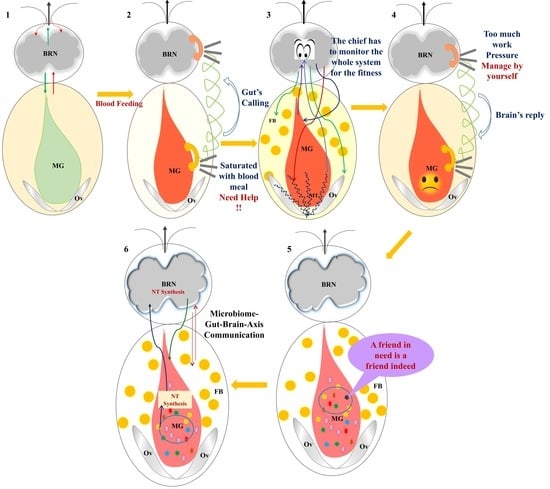
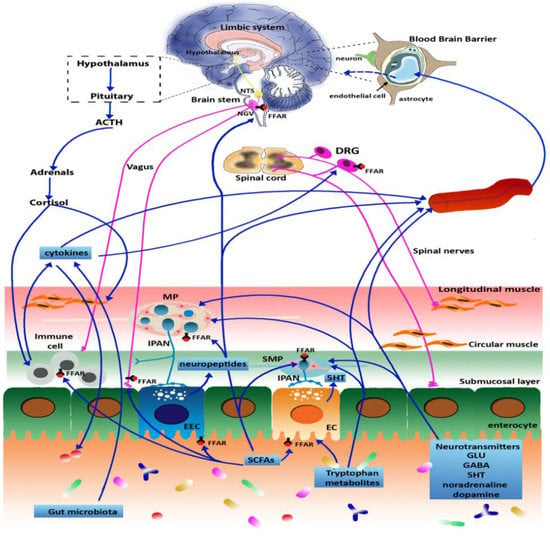
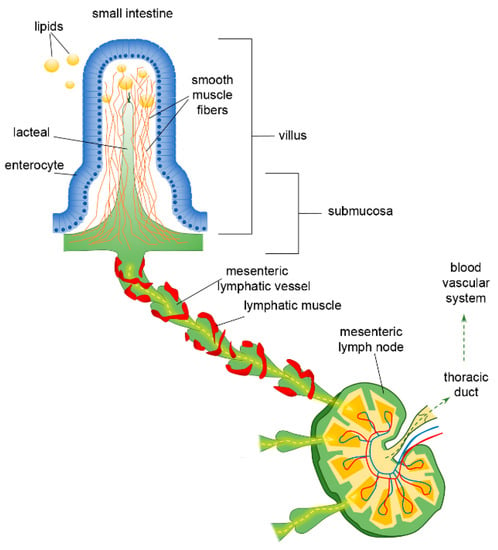
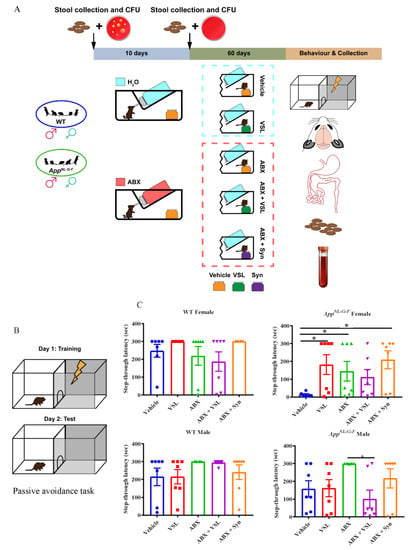
The complex bidirectional communication system existing between the gastrointestinal tract and the brain, termed the “microbiota-gut-brain axis”, has a pivotal role in sustaining the host’s homeostasis.
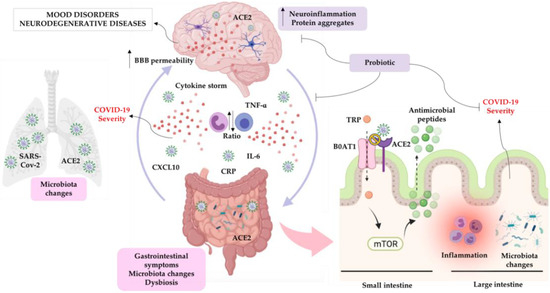
The large community of microorganisms embedding the gut and the host organism are now considered as composite and co-evolved organisms. In this context, the integration of signals deriving from the host neuronal, immune, and endocrine systems with signals deriving from the microbiota underlays the establishment and maintenance of health, as well as the development of disease states, both locally and in more distal brain regions.
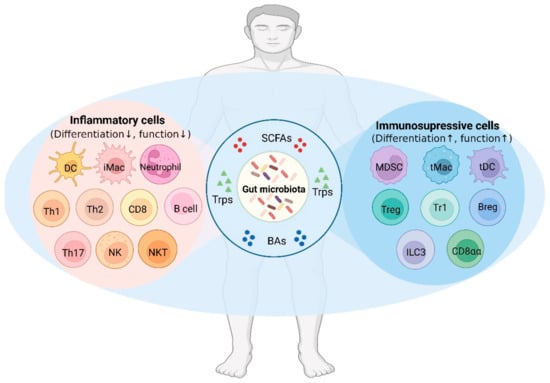
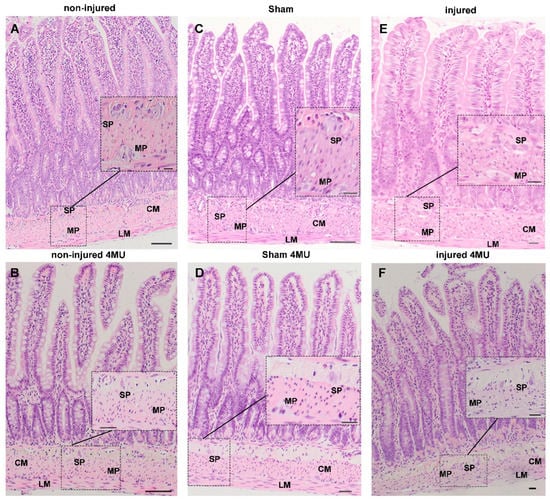
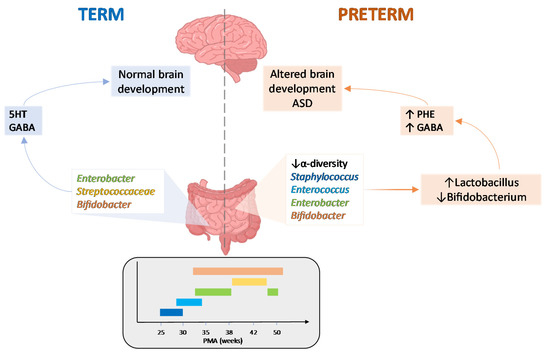
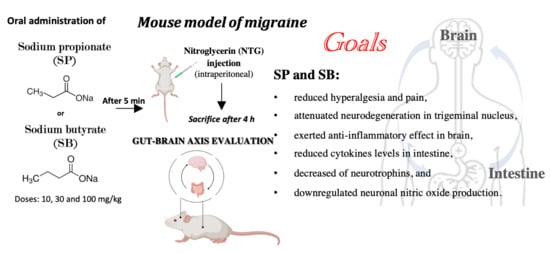
Among microbial metabolites, bile acids, short-chain fatty acids and tryptophan are detectable in different biological compartments, including feces and cerebrospinal fluid and exert important and diverse effects on host physiology, influencing the immune response, the integrity of the epithelial barrier to pathogen invasion, endocrine and neuronal functions giving rise to a microbiota-mediated bottom-up control of the central nervous system.
Research in this area opens the exciting possibility to target microbial metabolites to clarify the role of bacterial microbial flora in the pathogenesis of both gastrointestinal and brain disorders, as well as to discover new therapeutic strategies based on the administration of these “postbiotic” agents.
Dr. Cristina Giaroni
Dr. Andreina Baj
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- microbiota-gut-brain axis
- short-chain fatty acids
- microbiota-derived tryptophan metabolites
- secondary bile acids
- neuro-immune function
- microbiota-related gut disorders
- microbiota-related CNS disorders