Research Advances in Cellular Metabolism
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Metabolism".
Viewed by 4899
Share This Topical Collection
Editor
 Dr. Christian Neri
Dr. Christian Neri
 Dr. Christian Neri
Dr. Christian Neri
E-Mail
Website
Collection Editor
Centre National de la Recherche Scientifique (CNRS) and Sorbonne Université, Brain-C Lab, IBPS, Paris, France
Interests: neurodegenerative diseases; aging; systems biology; cellular models; extracellular vesicles
Topical Collection Information
Dear Colleagues,
This Topical Collection entitled “Research Advances in Cellular Metabolism” will collect high-quality research articles, communications, and review articles in cutting-edge fields of cellular metabolism. Since this Topical Collection aims to illustrate, through selected works, frontier research in cellular metabolism, we encourage Editorial Board Members of the Cellular Metabolism Section of Cells to contribute feature papers reflecting the latest progress in their research field, or to invite papers from relevant experts and colleagues.
We welcome manuscripts that emphasize the intracellular and extracellular regulatory mechanisms, cell type-dependent features, temporal features, functional effects and physiological role of cellular metabolism in development, maintenance, aging and disease. Relevant research topics include, but are not limited to, the following:
- Metabolic systems modelling
- Role of extracellular vesicles
- Role of phase transitions
- Genetic regulation
- Epigenetic regulation
- Epigenetic effects
- Cell type-specific features
- Temporal features
- Links with cellular homeostasis and function
- Links with organ metabolism and physiology
- Effects of environmental factors
- Epidemiology
- Biomarkers
- Therapeutic targets.
Dr. Christian Neri
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (3 papers)
2024
Open AccessReview
Kynurenines as a Novel Target for the Treatment of Inflammatory Disorders
by
Adrian Mor, Anna Tankiewicz-Kwedlo, Marianna Ciwun, Janina Lewkowicz and Dariusz Pawlak
Viewed by 1061
Abstract
This review discusses the potential of targeting the kynurenine pathway (KP) in the treatment of inflammatory diseases. The KP, responsible for the catabolism of the amino acid tryptophan (TRP), produces metabolites that regulate various physiological processes, including inflammation, cell cycle, and neurotransmission. These
[...] Read more.
This review discusses the potential of targeting the kynurenine pathway (KP) in the treatment of inflammatory diseases. The KP, responsible for the catabolism of the amino acid tryptophan (TRP), produces metabolites that regulate various physiological processes, including inflammation, cell cycle, and neurotransmission. These metabolites, although necessary to maintain immune balance, may accumulate excessively during inflammation, leading to systemic disorders. Key KP enzymes such as indoleamine 2,3-dioxygenase 1 (IDO1), indoleamine 2,3-dioxygenase 2 (IDO2), tryptophan 2,3-dioxygenase (TDO), and kynurenine 3-monooxygenase (KMO) have been considered promising therapeutic targets. It was highlighted that both inhibition and activation of these enzymes may be beneficial, depending on the specific inflammatory disorder. Several inflammatory conditions, including autoimmune diseases, for which modulation of KP activity holds therapeutic promise, have been described in detail. Preclinical studies suggest that this modulation may be an effective treatment strategy for diseases for which treatment options are currently limited. Taken together, this review highlights the importance of further research on the clinical application of KP enzyme modulation in the development of new therapeutic strategies for inflammatory diseases.
Full article
►▼
Show Figures
Open AccessArticle
Insights into PCSK9-LDLR Regulation and Trafficking via the Differential Functions of MHC-I Proteins HFE and HLA-C
by
Sepideh Mikaeeli, Ali Ben Djoudi Ouadda, Alexandra Evagelidis, Rachid Essalmani, Oscar Henrique Pereira Ramos, Carole Fruchart-Gaillard and Nabil G. Seidah
Cited by 1 | Viewed by 1359
Abstract
PCSK9 is implicated in familial hypercholesterolemia via targeting the cell surface PCSK9-LDLR complex toward lysosomal degradation. The M2 repeat in the PCSK9’s C-terminal domain is essential for its extracellular function, potentially through its interaction with an unidentified “protein X”. The M2 repeat was
[...] Read more.
PCSK9 is implicated in familial hypercholesterolemia via targeting the cell surface PCSK9-LDLR complex toward lysosomal degradation. The M2 repeat in the PCSK9’s C-terminal domain is essential for its extracellular function, potentially through its interaction with an unidentified “protein X”. The M2 repeat was recently shown to bind an R-x-E motif in MHC-class-I proteins (implicated in the immune system), like HLA-C, and causing their lysosomal degradation. These findings suggested a new role of PCSK9 in the immune system and that HLA-like proteins could be “protein X” candidates. However, the participation of each member of the MHC-I protein family in this process and their regulation of PCSK9’s function have yet to be determined. Herein, we compared the implication of MHC-I-like proteins such as HFE (involved in iron homeostasis) and HLA-C on the extracellular function of PCSK9. Our data revealed that the M2 domain regulates the intracellular sorting of the PCSK9-LDLR complex to lysosomes, and that HFE is a new target of PCSK9 that inhibits its activity on the LDLR, whereas HLA-C enhances its function. This work suggests the potential modulation of PCSK9’s functions through interactions of HFE and HLA-C.
Full article
►▼
Show Figures
Open AccessArticle
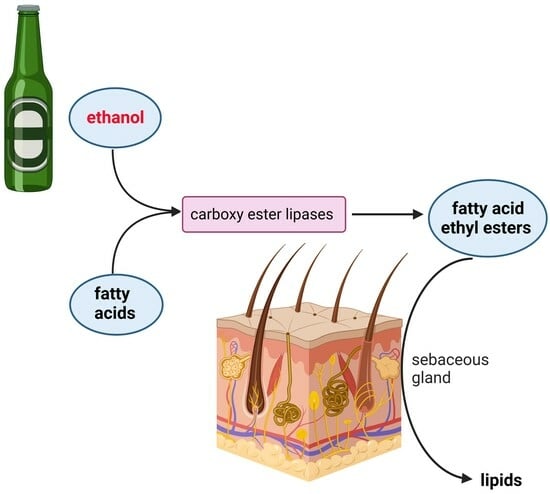
Alcohol Promotes Lipogenesis in Sebocytes—Implications for Acne
by
Johannes Kleemann, Jindrich Cinatl, Jr., Stephanie Hoffmann, Nadja Zöller, Deniz Özistanbullu, Christos C. Zouboulis, Roland Kaufmann and Stefan Kippenberger
Viewed by 1794
Abstract
The oral consumption of alcohol (ethanol) has a long tradition in humans and is an integral part of many cultures. The causal relationship between ethanol consumption and numerous diseases is well known. In addition to the well-described harmful effects on the liver and
[...] Read more.
The oral consumption of alcohol (ethanol) has a long tradition in humans and is an integral part of many cultures. The causal relationship between ethanol consumption and numerous diseases is well known. In addition to the well-described harmful effects on the liver and pancreas, there is also evidence that ethanol abuse triggers pathological skin conditions, including acne. In the present study, we addressed this issue by investigating the effect of ethanol on the energy metabolism in human SZ95 sebocytes, with particular focus on qualitative and quantitative lipogenesis. It was found that ethanol is a strong trigger for lipogenesis, with moderate effects on cell proliferation and toxicity. We identified the non-oxidative metabolism of ethanol, which produced fatty acid ethyl esters (FAEEs), as relevant for the lipogenic effect—the oxidative metabolism of ethanol does not contribute to lipogenesis. Correspondingly, using the Seahorse extracellular flux analyzer, we found an inhibition of the mitochondrial oxygen consumption rate as a measure of mitochondrial ATP production by ethanol. The ATP production rate from glycolysis was not affected. These data corroborate that ethanol-induced lipogenesis is independent from oxygen. In sum, our results give a causal explanation for the prevalence of acne in heavy drinkers, confirming that alcoholism should be considered as a systemic disease. Moreover, the identification of key factors driving ethanol-dependent lipogenesis may also be relevant in the treatment of acne vulgaris.
Full article
►▼
Show Figures