Molecular and Cellular Mechanisms of Parkinson's Disease
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Aging".
Viewed by 58103Editor
Interests: botulinum toxin; dystonia; cervical dystonia; sialorrhea; Parkinson’s disease; Blepharospasm
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
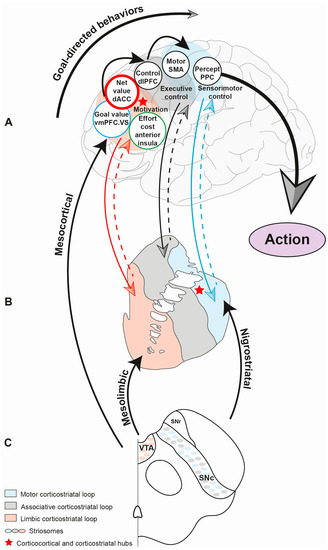
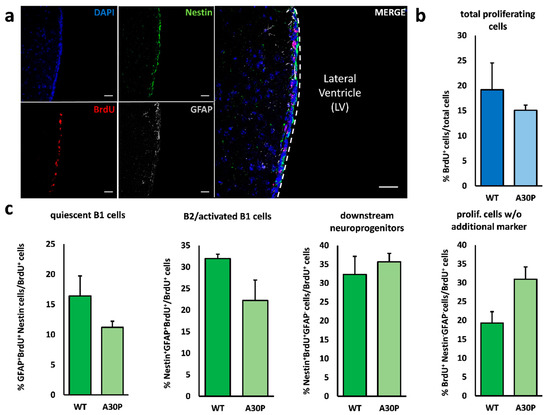
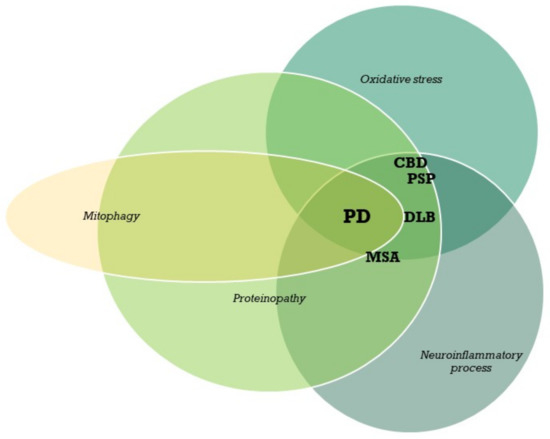
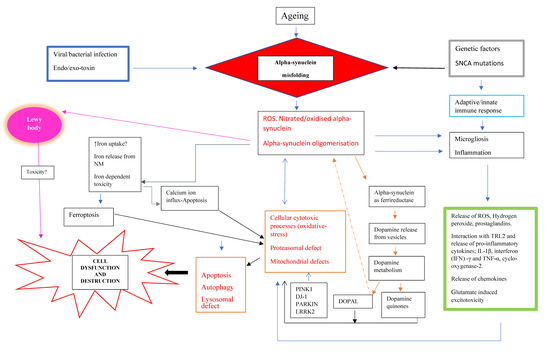
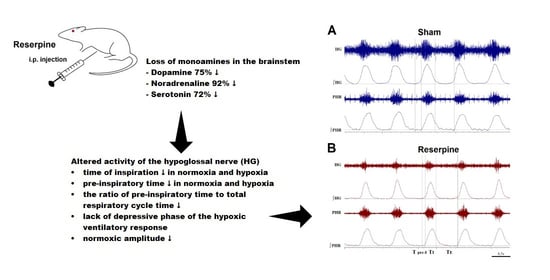
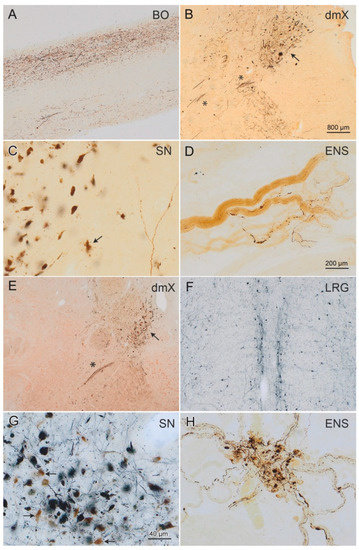
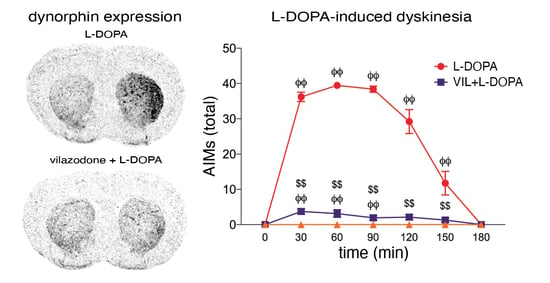
Parkinson’s disease is a disease for which we have attained a massive gain in knowledge within the last few decades, and for which the number of publications has increased as in hardly any other area of medicine. We are fortunate to have many effective medications at our disposal for this disease. The central issue here has been the substantia nigra, and the deficiency in levodopa has been the pivotal focus for therapy. In the last two decades, however, we have learned that emphasis on these concepts offers only limited benefits and may in fact be detrimental for further progress in research. To be precise, the substantia nigra is neither the origin nor the end point of the degenerative process, and furthermore dopaminergic therapy is merely a symptomatic intervention. Of course, all further progress is a factor of our basic hypotheses, but these have to be substantiated as to their validity. In Parkinson’s diagnostics and therapy, we have arrived at something of a stalemate: many new findings are truly spectacular, but not yet the final answer to all our questions. As clinicians, of course, we tend to target quick solutions, but so do our patients, and these quick solutions are still very illusive. For this reason, we have to return to the situation at the historical start of work on Parkinson’s and take a good look at current gaps in knowledge in order to work them out scientifically. For this reason, we have decided to put out a Topical Collection entitled “Molecular and Cellular Mechanisms of Parkinson’s Disease”. We are deliberately not working on a new and comprehensive model in this Topical Collection, but intend to outline the current status of different aspects so that the large spectrum of findings can be seen and so that some hints can be gleaned as to just where we might profitably pursue new fields of research and where we should avoid some potential dead-ends. Put metaphorically, if I have become lost in a city it is more helpful to return to the starting point and not just to rely on keeping to the obviously wrong route taken. So, we want to meet at the market and trade our current awareness.
Prof. Wolfgang Jost
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Parkinson’s disease
- Alpha-synuclein
- Genetics
- Inflammation