Cilia and Flagella: Structure, Function and Beyond
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Intracellular and Plasma Membranes".
Viewed by 131018Editors
Interests: centrosome; cilia; cancer; ciliopathy; cell cycle; cell division; microtubule; cell biology; molecular biology; biochemistry; genetics
Special Issues, Collections and Topics in MDPI journals
Interests: structural biology; vesicular traffic; cilium biogenesis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
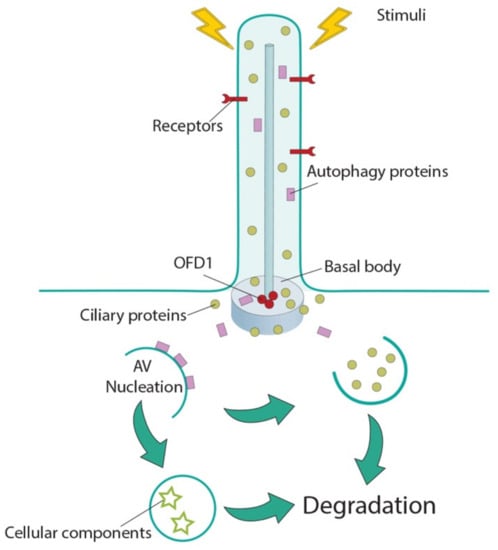
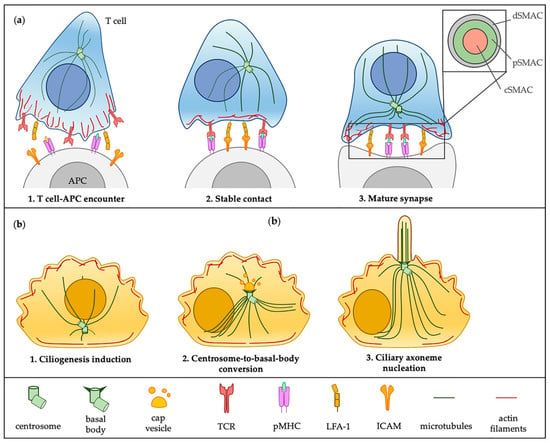
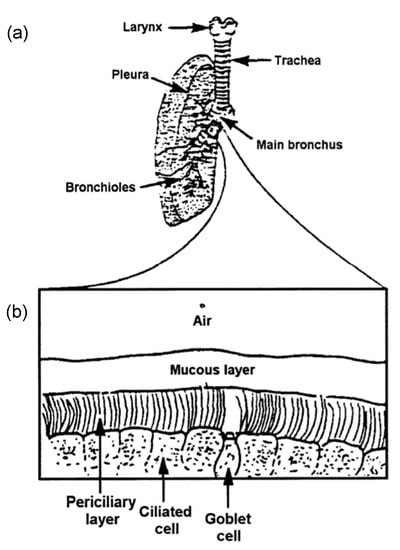
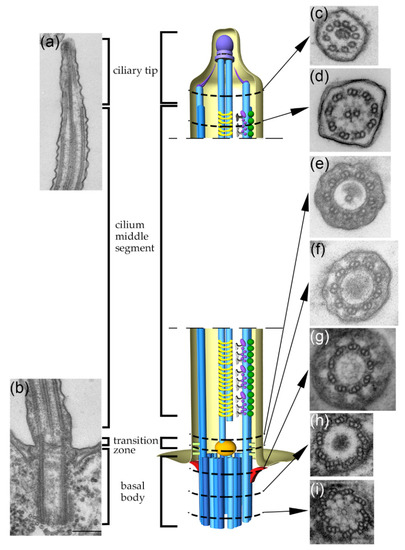
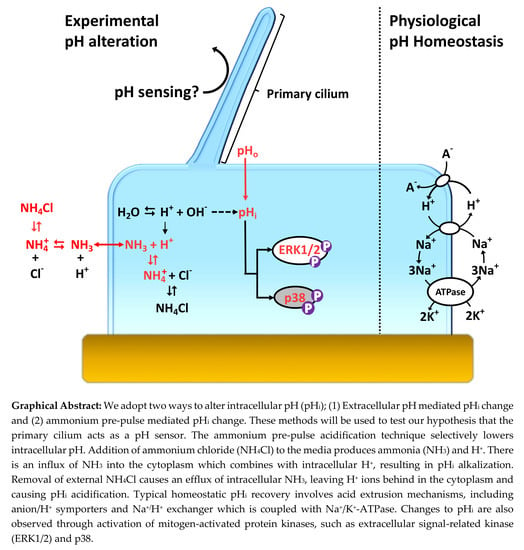
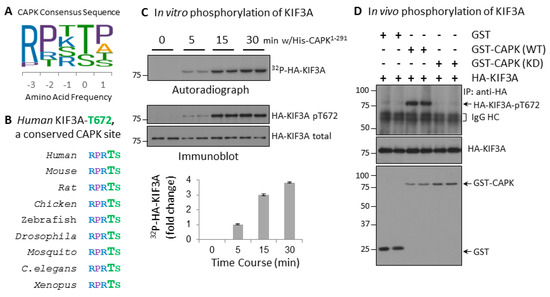
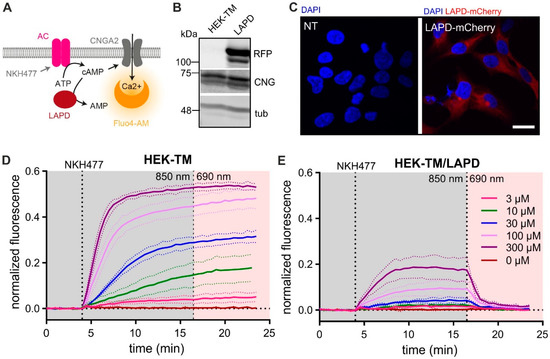
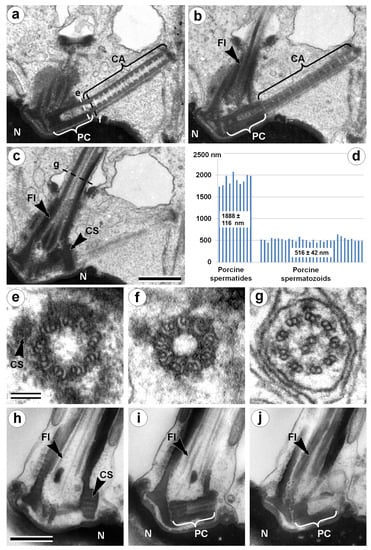
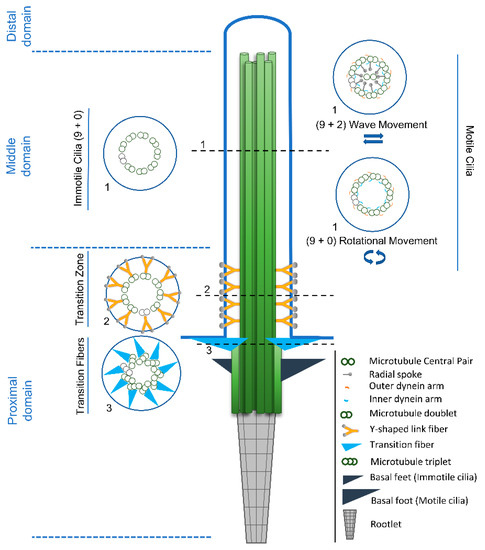
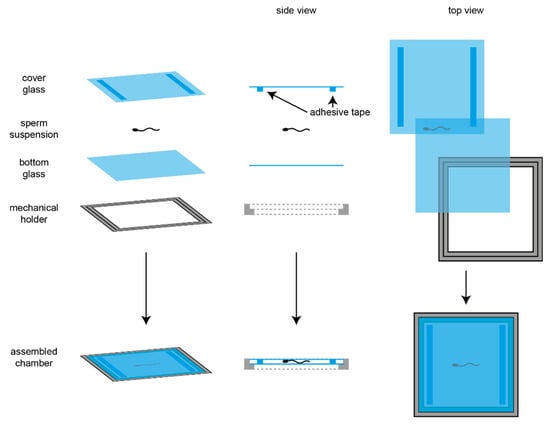
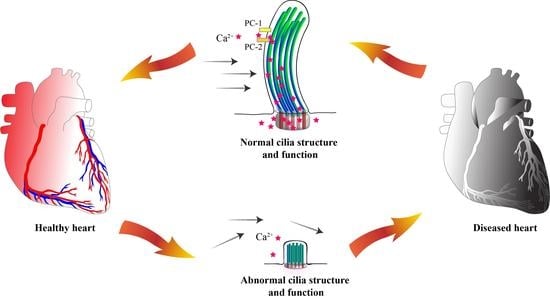
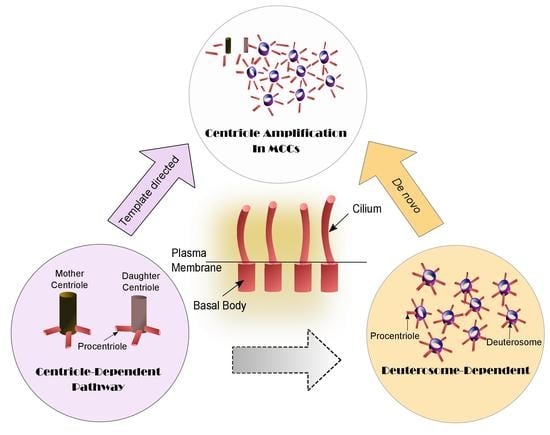
Cilia and flagella are antenna-like protrusions present on many types of eukaryotic cells. Consequently, they have evolved to perform diverse functions, such as locomotion, mucus clearance, fluid circulation, chemosensation, and mechanosensation. It is now known that defects in cilia and flagella assembly or function give rise to a wide spectrum of human diseases including infertility, loss of vision, kidney cysts, respiratory defects, skeletal anomalies, and neurological disorders. In spite of functional differences, cilia and flagella are remarkably similar in terms of molecular composition and structure, consisting of stabilized microtubules arranged in a nine-fold radial symmetry. Cilia and flagella were originally discovered centuries ago, yet many aspects regarding their structure and function are just beginning to emerge, thanks in part to breakthrough technologies, such as super-resolution microscopy, cryo-electron microscopy, and BioID. This Topical Collection focuses on current advances in the biology of cilia and flagella. We welcome contributions that include, but are not limited to, structural and functional studies of cilia/flagella, different cilia/flagella model systems, mechanisms of cilia/flagella assembly, maintenance, and disassembly, mechanisms of cilia/flagella-mediated sensing mechanisms and signal transduction, link between cilia/flagella and the cell cycle or other relevant cell physiological processes implicated in disease. Submitted articles will be peer reviewed in accordance with the journal’s policy. The Collection Editors declare no competing interests.
Dr. William Tsang
Dr. Gang Dong
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cilia
- flagella
- centrioles
- basal bodies
- centrosomes
- axonemes
- microtubules
- ciliogenesis
- ciliopathies