Recent Advances in Oncology Nanomedicine: Toward Novel Personalized Medicine Tools to Fight Human Cancer
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Biophysics".
Viewed by 53430
Share This Topical Collection
Editor
Topical Collection Information
Dear Colleagues,
Cancer continues to be one of the most difficult global healthcare problems. Although there is a large spectrum of drugs that can be used in therapy, the main problem is selectively killing cancer cells while reducing collateral toxicity to healthy cells. There are several biological barriers to effective drug delivery in cancer such as brain, renal, hepatic, or immune clearance. Nanoparticles loaded with drugs can be designed to overcome these biological barriers to improve efficacy while reducing morbidity. In this research avenue, next-generation nanomedicines need to be better targeted to specifically destroy cancerous tissue but face several obstacles in their clinical development, including the identification of appropriate biomarkers to target, the scale-up of synthesis, and the need to produce a reproducible effect. These hurdles need to be overcome through multidisciplinary collaborations across academia, the pharmaceutical industry, and clinicians in order to achieve the goal of eradicating cancer.
This Topical Collection of Cells is therefore dedicated to collecting multidisciplinary investigations covering cell biology and physiology, molecular biology, and biophysics expertise to provide innovative experimental preclinical proposals to the challenges of development of nanomedicines for cancer.
Dr. Sergio Comincini
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- Extracellular vesicle
- Nanoparticles
- Therapeutics
- Combination treatment
- Nanobiomaterials
- Translational oncology
Published Papers (12 papers)
Open AccessReview
Boron Vehiculating Nanosystems for Neutron Capture Therapy in Cancer Treatment
by
Giorgia Ailuno, Alice Balboni, Gabriele Caviglioli, Francesco Lai, Federica Barbieri, Irene Dellacasagrande, Tullio Florio and Sara Baldassari
Cited by 19 | Viewed by 3149
Abstract
Boron neutron capture therapy is a low-invasive cancer therapy based on the neutron fission process that occurs upon thermal neutron irradiation of
10B-containing compounds; this process causes the release of alpha particles that selectively damage cancer cells. Although several clinical studies involving
[...] Read more.
Boron neutron capture therapy is a low-invasive cancer therapy based on the neutron fission process that occurs upon thermal neutron irradiation of
10B-containing compounds; this process causes the release of alpha particles that selectively damage cancer cells. Although several clinical studies involving mercaptoundecahydro-
closo-dodecaborate and the boronophenylalanine–fructose complex are currently ongoing, the success of this promising anticancer therapy is hampered by the lack of appropriate drug delivery systems to selectively carry therapeutic concentrations of boron atoms to cancer tissues, allowing prolonged boron retention therein and avoiding the damage of healthy tissues. To achieve these goals, numerous research groups have explored the possibility to formulate nanoparticulate systems for boron delivery. In this review. we report the newest developments on boron vehiculating drug delivery systems based on nanoparticles, distinguished on the basis of the type of carrier used, with a specific focus on the formulation aspects.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
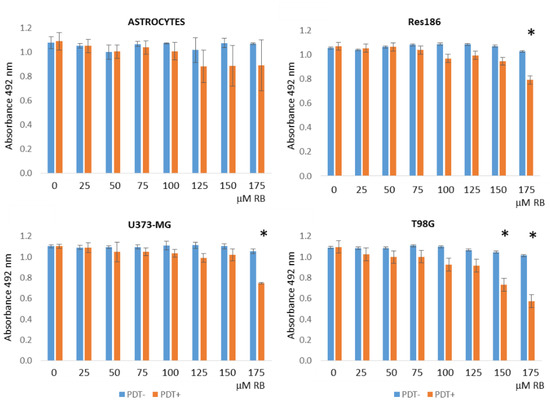
Enhanced Delivery of Rose Bengal by Amino Acids Starvation and Exosomes Inhibition in Human Astrocytoma Cells to Potentiate Anticancer Photodynamic Therapy Effects
by
Bianca Slivinschi, Federico Manai, Carolina Martinelli, Francesca Carriero, Camilla D’Amato, Martina Massarotti, Giorgia Bresciani, Claudio Casali, Gloria Milanesi, Laura Artal, Lisa Zanoletti, Federica Milella, Davide Arfini, Alberto Azzalin, Sara Demartis, Elisabetta Gavini and Sergio Comincini
Cited by 9 | Viewed by 3366
Abstract
Photodynamic therapy (PDT) is a promising anticancer strategy based on the light energy stimulation of photosensitizers (PS) molecules within a malignant cell. Among a multitude of recently challenged PS, Rose bengal (RB) has been already reported as an inducer of cytotoxicity in different
[...] Read more.
Photodynamic therapy (PDT) is a promising anticancer strategy based on the light energy stimulation of photosensitizers (PS) molecules within a malignant cell. Among a multitude of recently challenged PS, Rose bengal (RB) has been already reported as an inducer of cytotoxicity in different tumor cells. However, RB displays a low penetration capability across cell membranes. We have therefore developed a short-term amino acids starvation protocol that significantly increases RB uptake in human astrocytoma cells compared to normal rat astrocytes. Following induced starvation uptake, RB is released outside cells by the exocytosis of extracellular vesicles (EVs). Thus, we have introduced a specific pharmacological treatment, based on the GW4869 exosomes inhibitor, to interfere with RB extracellular release. These combined treatments allow significantly reduced nanomolar amounts of administered RB and a decrease in the time interval required for PDT stimulation. The overall conditions affected astrocytoma viability through the activation of apoptotic pathways. In conclusion, we have developed for the first time a combined scheme to simultaneously increase the RB uptake in human astrocytoma cells, reduce the extracellular release of the drug by EVs, and improve the effectiveness of PDT-based treatments. Importantly, this strategy might be a valuable approach to efficiently deliver other PS or chemotherapeutic drugs in tumor cells.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
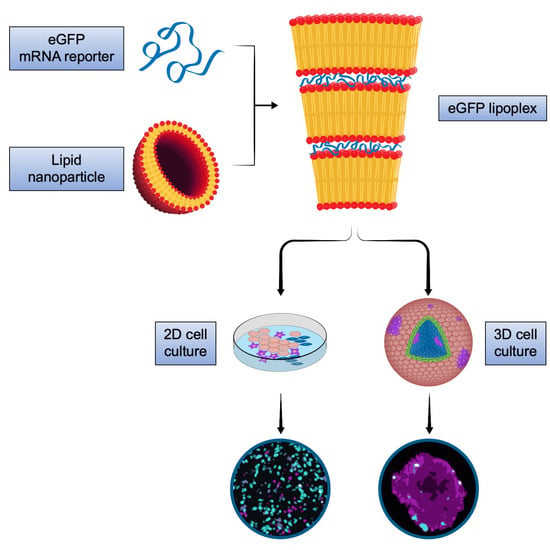
3D Melanoma Cocultures as Improved Models for Nanoparticle-Mediated Delivery of RNA to Tumors
by
Maximilian E. A. Schäfer, Florian Keller, Jens Schumacher, Heinrich Haas, Fulvia Vascotto, Ugur Sahin, Mathias Hafner and Rüdiger Rudolf
Cited by 6 | Viewed by 3443
Abstract
Cancer therapy is an emergent application for mRNA therapeutics. While in tumor immunotherapy, mRNA encoding for tumor-associated antigens is delivered to antigen-presenting cells in spleen and lymph nodes, other therapeutic options benefit from immediate delivery of mRNA nanomedicines directly to the tumor. However,
[...] Read more.
Cancer therapy is an emergent application for mRNA therapeutics. While in tumor immunotherapy, mRNA encoding for tumor-associated antigens is delivered to antigen-presenting cells in spleen and lymph nodes, other therapeutic options benefit from immediate delivery of mRNA nanomedicines directly to the tumor. However, tumor targeting of mRNA therapeutics is still a challenge, since, in addition to delivery of the cargo to the tumor, specifics of the targeted cell type as well as its interplay with the tumor microenvironment are crucial for successful intervention. This study investigated lipoplex nanoparticle-mediated mRNA delivery to spheroid cell culture models of melanoma. Insights into cell-type specific targeting, non-cell-autonomous effects, and penetration capacity in tumor and stroma cells of the mRNA lipoplex nanoparticles were obtained. It was shown that both coculture of different cell types as well as three-dimensional cell growth characteristics can modulate distribution and transfection efficiency of mRNA lipoplex formulations. The results demonstrate that three-dimensional coculture spheroids can provide a valuable surplus of information in comparison to adherent cells. Thus, they may represent in vitro models with enhanced predictivity for the in vivo activity of cancer nanotherapeutics.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Fostering “Education”: Do Extracellular Vesicles Exploit Their Own Delivery Code?
by
Mayra Paolillo, Sergio Comincini and Sergio Schinelli
Cited by 4 | Viewed by 3741
Abstract
Extracellular vesicles (EVs), comprising large microvesicles (MVs) and exosomes (EXs), play a key role in intercellular communication, both in physiological and in a wide variety of pathological conditions. However, the education of EV target cells has so far mainly been investigated as a
[...] Read more.
Extracellular vesicles (EVs), comprising large microvesicles (MVs) and exosomes (EXs), play a key role in intercellular communication, both in physiological and in a wide variety of pathological conditions. However, the education of EV target cells has so far mainly been investigated as a function of EX cargo, while few studies have focused on the characterization of EV surface membrane molecules and the mechanisms that mediate the addressability of specific EVs to different cell types and tissues. Identifying these mechanisms will help fulfill the diagnostic, prognostic, and therapeutic promises fueled by our growing knowledge of EVs. In this review, we first discuss published studies on the presumed EV “delivery code” and on the combinations of the hypothesized EV surface membrane “sender” and “recipient” molecules that may mediate EV targeting in intercellular communication. Then we briefly review the main experimental approaches and techniques, and the bioinformatic tools that can be used to identify and characterize the structure and functional role of EV surface membrane molecules. In the final part, we present innovative techniques and directions for future research that would improve and deepen our understandings of EV-cell targeting.
Full article
►▼
Show Figures
Open AccessReview
Nano-Strategies Targeting the Integrin αvβ3 Network for Cancer Therapy
by
Tsai-Mu Cheng, Wong-Jin Chang, Hsiu-Yi Chu, Roberto De Luca, Jens Z. Pedersen, Sandra Incerpi, Zi-Lin Li, Ya-Jung Shih, Hung-Yun Lin, Kuan Wang and Jacqueline Whang-Peng
Cited by 48 | Viewed by 5180
Abstract
Integrin αvβ3, a cell surface receptor, participates in signaling transduction pathways in cancer cell proliferation and metastasis. Several ligands bind to integrin αvβ3 to regulate proliferation and metastasis in cancer cells. Crosstalk between the integrin and other signal transduction pathways also plays an
[...] Read more.
Integrin αvβ3, a cell surface receptor, participates in signaling transduction pathways in cancer cell proliferation and metastasis. Several ligands bind to integrin αvβ3 to regulate proliferation and metastasis in cancer cells. Crosstalk between the integrin and other signal transduction pathways also plays an important role in modulating cancer proliferation. Carcinoembryonic antigen cell adhesion molecule 6 (CEACAM6) activates the downstream integrin FAK to stimulate biological activities including cancer proliferation and metastasis. Blockage of signals related to integrin αvβ3 was shown to be a promising target for cancer therapies. 3,3′,5,5′-tetraiodothyroacetic acid (tetrac) completely binds to the integrin with the thyroid hormone to suppress cancer proliferation. The (E)-stilbene analog, resveratrol, also binds to integrin αvβ3 to inhibit cancer growth. Recently, nanotechnologies have been used in the biomedical field for detection and therapeutic purposes. In the current review, we show and evaluate the potentiation of the nanomaterial carrier RGD peptide, derivatives of PLGA-tetrac (NDAT), and nanoresveratrol targeting integrin αvβ3 in cancer therapies.
Full article
►▼
Show Figures
Open AccessArticle
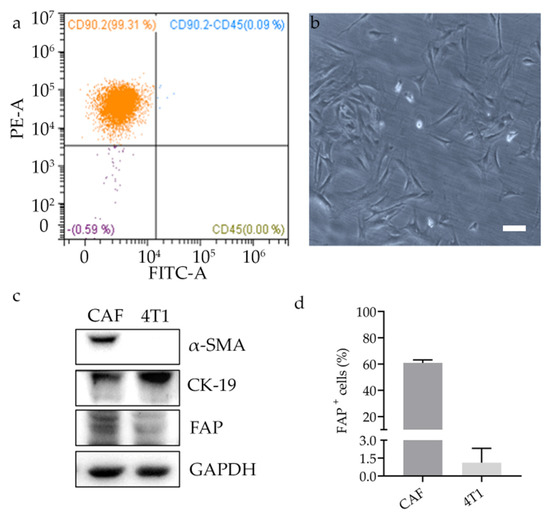
Selective Targeting of Cancer-Associated Fibroblasts by Engineered H-Ferritin Nanocages Loaded with Navitoclax
by
Leopoldo Sitia, Arianna Bonizzi, Serena Mazzucchelli, Sara Negri, Cristina Sottani, Elena Grignani, Maria Antonietta Rizzuto, Davide Prosperi, Luca Sorrentino, Carlo Morasso, Raffaele Allevi, Marta Sevieri, Filippo Silva, Marta Truffi and Fabio Corsi
Cited by 31 | Viewed by 4739
Abstract
Cancer-associated fibroblasts (CAFs) are key actors in regulating cancer progression. They promote tumor growth, metastasis formation, and induce drug resistance. For these reasons, they are emerging as potential therapeutic targets. Here, with the aim of developing CAF-targeted drug delivery agents, we functionalized H-ferritin
[...] Read more.
Cancer-associated fibroblasts (CAFs) are key actors in regulating cancer progression. They promote tumor growth, metastasis formation, and induce drug resistance. For these reasons, they are emerging as potential therapeutic targets. Here, with the aim of developing CAF-targeted drug delivery agents, we functionalized H-ferritin (HFn) nanocages with fibroblast activation protein (FAP) antibody fragments. Functionalized nanocages (HFn-FAP) have significantly higher binding with FAP
+ CAFs than with FAP
− cancer cells. We loaded HFn-FAP with navitoclax (Nav), an experimental Bcl-2 inhibitor pro-apoptotic drug, whose clinical development is limited by its strong hydrophobicity and toxicity. We showed that Nav is efficiently loaded into HFn (HNav), maintaining its mechanism of action. Incubating Nav-loaded functionalized nanocages (HNav-FAP) with FAP
+ cells, we found significantly higher cytotoxicity as compared to non-functionalized HNav. This was correlated with a significantly higher drug release only in FAP
+ cells, confirming the specific targeting ability of functionalized HFn. Finally, we showed that HFn-FAP is able to reach the tumor and to target CAFs in a mouse syngeneic model of triple negative breast cancer after intravenous administration. Our data show that HNav-FAP could be a promising tool to enhance specific drug delivery into CAFs, thus opening new therapeutic possibilities focused on tumor microenvironment.
Full article
►▼
Show Figures
Open AccessReview
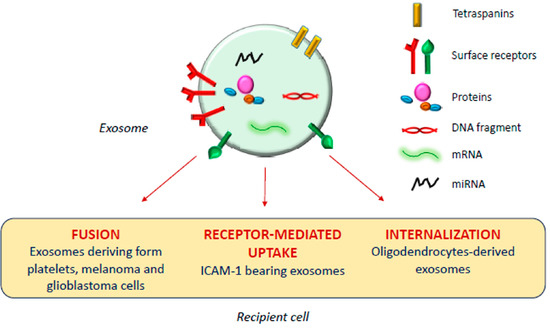
Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research
by
Giorgia Ailuno, Sara Baldassari, Francesco Lai, Tullio Florio and Gabriele Caviglioli
Cited by 56 | Viewed by 7992
Abstract
Exosomes are endosome-derived nanovesicles produced by healthy as well as diseased cells. Their proteic, lipidic and nucleic acid composition is related to the cell of origin, and by vehiculating bioactive molecules they are involved in cell-to-cell signaling, both in healthy and pathologic conditions.
[...] Read more.
Exosomes are endosome-derived nanovesicles produced by healthy as well as diseased cells. Their proteic, lipidic and nucleic acid composition is related to the cell of origin, and by vehiculating bioactive molecules they are involved in cell-to-cell signaling, both in healthy and pathologic conditions. Being nano-sized, non-toxic, biocompatible, scarcely immunogenic, and possessing targeting ability and organotropism, exosomes have been proposed as nanocarriers for their potential application in diagnosis and therapy. Among the different techniques exploited for exosome isolation, the sequential ultracentrifugation/ultrafiltration method seems to be the gold standard; alternatively, commercially available kits for exosome selective precipitation from cell culture media are frequently employed. To load a drug or a detectable agent into exosomes, endogenous or exogenous loading approaches have been developed, while surface engineering procedures, such as click chemistry, hydrophobic insertion and exosome display technology, allow for obtaining actively targeted exosomes. This review reports on diagnostic or theranostic platforms based on exosomes or exosome-mimetic vesicles, highlighting the diverse preparation, loading and surface modification methods applied, and the results achieved so far.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
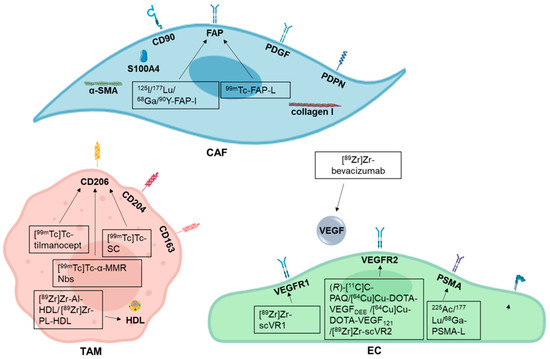
Development of Radiotracers for Breast Cancer—The Tumor Microenvironment as an Emerging Target
by
Amelie Heesch, Jochen Maurer, Elmar Stickeler, Mohsen Beheshti, Felix M. Mottaghy and Agnieszka Morgenroth
Cited by 14 | Viewed by 4367
Abstract
Molecular imaging plays an increasingly important role in the diagnosis and treatment of different malignancies. Radiolabeled probes enable the visualization of the primary tumor as well as the metastases and have been also employed in targeted therapy and theranostic approaches. With breast cancer
[...] Read more.
Molecular imaging plays an increasingly important role in the diagnosis and treatment of different malignancies. Radiolabeled probes enable the visualization of the primary tumor as well as the metastases and have been also employed in targeted therapy and theranostic approaches. With breast cancer being the most common malignancy in women worldwide it is of special interest to develop novel targeted treatments. However, tumor microenvironment and escape mechanisms often limit their therapeutic potential. Addressing tumor stroma associated targets provides a promising option to inhibit tumor growth and angiogenesis and to disrupt tumor tissue architecture. This review describes recent developments on radiolabeled probes used in diagnosis and treatment of breast cancer especially in triple negative type with the focus on potential targets offered by the tumor microenvironment, like tumor associated macrophages, cancer associated fibroblasts, and endothelial cells.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
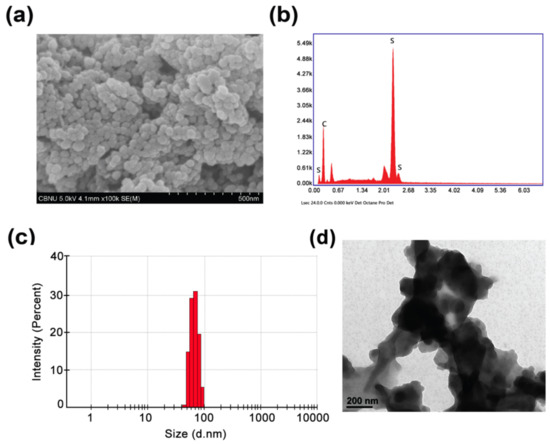
NIR-Triggered Hyperthermal Effect of Polythiophene Nanoparticles Synthesized by Surfactant-Free Oxidative Polymerization Method on Colorectal Carcinoma Cells
by
Deval Prasad Bhattarai and Beom Su Kim
Cited by 13 | Viewed by 2701
Abstract
In this work, polythiophene nanoparticles (PTh–NPs) were synthesized by a surfactant-free oxidative chemical polymerization method at 60 °C, using ammonium persulphate as an oxidant. Various physicochemical properties were studied in terms of field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Fourier transform
[...] Read more.
In this work, polythiophene nanoparticles (PTh–NPs) were synthesized by a surfactant-free oxidative chemical polymerization method at 60 °C, using ammonium persulphate as an oxidant. Various physicochemical properties were studied in terms of field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD), Fourier transform infra-red (FT-IR) spectroscopy, and differential scanning calorimetry (DSC)/thermogravimetric analysis (TGA). Photothermal performance of the as-synthesized PTh–NPs was studied by irradiating near infra-red of 808 nm under different concentration of the substrate and power supply. The photothermal stability of PTh–NPs was also studied. Photothermal effects of the as-synthesized PTh–NPs on colorectal cancer cells (CT-26) were studied at 100 µg/mL concentration and 808 nm NIR irradiation of 2.0 W/cm
2 power. Our in vitro results showed remarkable NIR laser-triggered photothermal apoptotic cell death by PTh–NPs. Based on the experimental findings, it is revealed that PTh–NPs can act as a heat mediator and can be an alternative material for photothermal therapy in cancer treatment.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
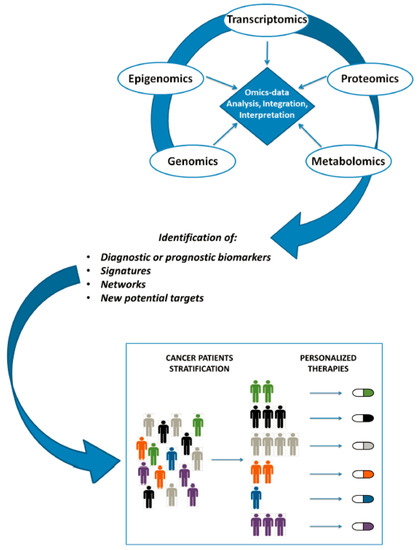
Precision Medicine: Steps along the Road to Combat Human Cancer
by
Samuel F. Nassar, Khadir Raddassi, Baljit Ubhi, Joseph Doktorski and Ahmad Abulaban
Cited by 32 | Viewed by 5597
Abstract
The diagnosis and treatment of diseases such as cancer is becoming more accurate and specialized with the advent of precision medicine techniques, research and treatments. Reaching down to the cellular and even sub-cellular level, diagnostic tests can pinpoint specific, individual information from each
[...] Read more.
The diagnosis and treatment of diseases such as cancer is becoming more accurate and specialized with the advent of precision medicine techniques, research and treatments. Reaching down to the cellular and even sub-cellular level, diagnostic tests can pinpoint specific, individual information from each patient, and guide providers to a more accurate plan of treatment. With this advanced knowledge, researchers and providers can better gauge the effectiveness of drugs, radiation, and other therapies, which is bound to lead to a more accurate, if not more positive, prognosis. As precision medicine becomes more established, new techniques, equipment, materials and testing methods will be required. Herein, we will examine the recent innovations in assays, devices and software, along with next generation sequencing in genomics diagnostics which are in use or are being developed for personalized medicine. So as to avoid duplication and produce the fullest possible benefit, all involved must be strongly encouraged to collaborate, across national borders, public and private sectors, science, medicine and academia alike. In this paper we will offer recommendations for tools, research and development, along with ideas for implementation. We plan to begin with discussion of the lessons learned to date, and the current research on pharmacogenomics. Given the steady stream of advances in imaging mass spectrometry and nanoLC-MS/MS, and use of genomic, proteomic and metabolomics biomarkers to distinguish healthy tissue from diseased cells, there is great potential to utilize pharmacogenomics to tailor a drug or drugs to a particular cohort of patients. Such efforts very well may bring increased hope for small groups of non-responders and those who have demonstrated adverse reactions to current treatments.
Full article
►▼
Show Figures
Open AccessArticle
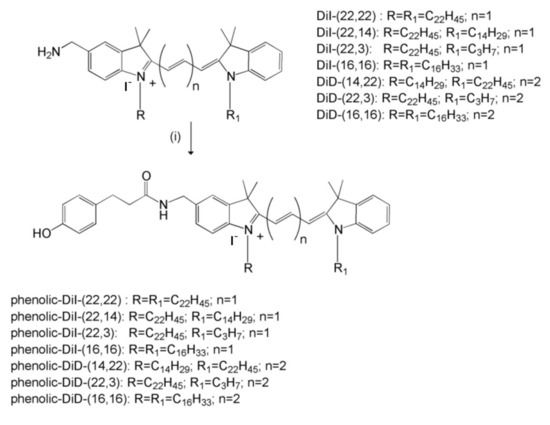
Biotherapy of Brain Tumors with Phosphatidylserine-Targeted Radioiodinated SapC-DOPS Nanovesicles
by
Harold W. Davis, Subrahmanya D. Vallabhapurapu, Zhengtao Chu, Michael A. Wyder, Kenneth D. Greis, Venette Fannin, Ying Sun, Pankaj B. Desai, Koon Y. Pak, Brian D. Gray and Xiaoyang Qi
Cited by 6 | Viewed by 3016
Abstract
Glioblastoma multiforme (GBM), a common type of brain cancer, has a very poor prognosis. In general, viable GBM cells exhibit elevated phosphatidylserine (PS) on their membrane surface compared to healthy cells. We have developed a drug, saposin C-dioleoylphosphatidylserine (SapC-DOPS), that selectively targets cancer
[...] Read more.
Glioblastoma multiforme (GBM), a common type of brain cancer, has a very poor prognosis. In general, viable GBM cells exhibit elevated phosphatidylserine (PS) on their membrane surface compared to healthy cells. We have developed a drug, saposin C-dioleoylphosphatidylserine (SapC-DOPS), that selectively targets cancer cells by honing in on this surface PS. To examine whether SapC-DOPS, a stable, blood–brain barrier-penetrable nanovesicle, could be an effective delivery system for precise targeted therapy of radiation, we iodinated several carbocyanine-based fluorescent reporters with either stable iodine (
127I) or radioactive isotopes (
125I and
131I). While all of the compounds, when incorporated into the SapC-DOPS delivery system, were taken up by human GBM cell lines, we chose the two that best accumulated in the cells (DiI (22,3) and DiD (16,16)). Pharmacokinetics were conducted with
125I-labeled compounds and indicated that DiI (22,3)-SapC-DOPS had a time to peak in the blood of 0.66 h and an elimination half-life of 8.4 h. These values were 4 h and 11.5 h, respectively, for DiD (16,16)-SapC-DOPS. Adult nude mice with GBM cells implanted in their brains were treated with
131I-DID (16,16)-SapC-DOPS. Mice receiving the radionuclide survived nearly 50% longer than the control groups. These data suggest a potential novel, personalized treatment for a devastating brain disease.
Full article
►▼
Show Figures
Open AccessFeature PaperEditor’s ChoiceArticle
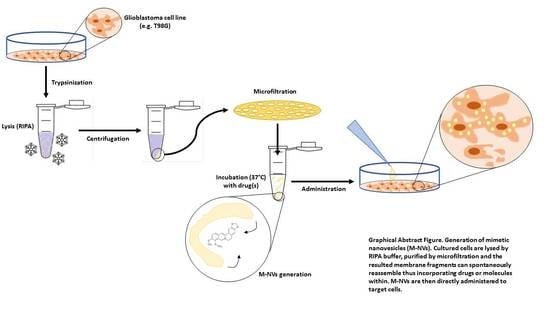
Development of Artificial Plasma Membranes Derived Nanovesicles Suitable for Drugs Encapsulation
by
Carolina Martinelli, Fabio Gabriele, Elena Dini, Francesca Carriero, Giorgia Bresciani, Bianca Slivinschi, Marco Dei Giudici, Lisa Zanoletti, Federico Manai, Mayra Paolillo, Sergio Schinelli, Alberto Azzalin and Sergio Comincini
Cited by 17 | Viewed by 4202
Abstract
Extracellular vesicles (EVs) are considered as promising nanoparticle theranostic tools in many pathological contexts. The increasing clinical employment of therapeutic nanoparticles is contributing to the development of a new research area related to the design of artificial EVs. To this aim, different approaches
[...] Read more.
Extracellular vesicles (EVs) are considered as promising nanoparticle theranostic tools in many pathological contexts. The increasing clinical employment of therapeutic nanoparticles is contributing to the development of a new research area related to the design of artificial EVs. To this aim, different approaches have been described to develop mimetic biologically functional nanovescicles. In this paper, we suggest a simplified procedure to generate plasma membrane-derived nanovesicles with the possibility to efficiently encapsulate different drugs during their spontaneously assembly. After physical and molecular characterization by Tunable Resistive Pulse Sensing (TRPS) technology, transmission electron microscopy, and flow cytometry, as a proof of principle, we have loaded into mimetic EVs the isoquinoline alkaloid Berberine chloride and the chemotherapy compounds Temozolomide or Givinostat. We demonstrated the fully functionality of these nanoparticles in drug encapsulation and cell delivery, showing, in particular, a similar cytotoxic effect of direct cell culture administration of the anticancer drugs. In conclusion, we have documented the possibility to easily generate scalable nanovesicles with specific therapeutic cargo modifications useful in different drug delivery contexts.
Full article
►▼
Show Figures