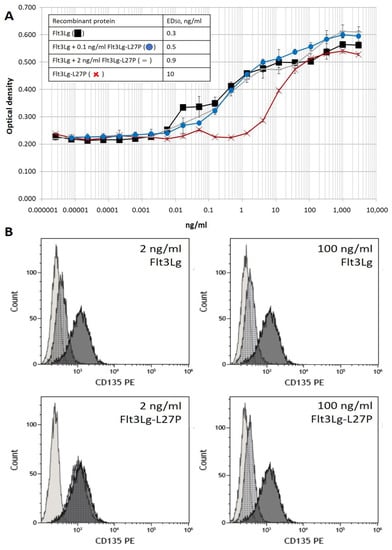
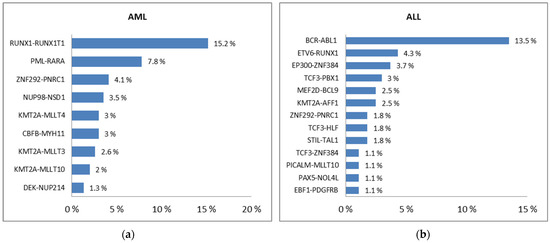
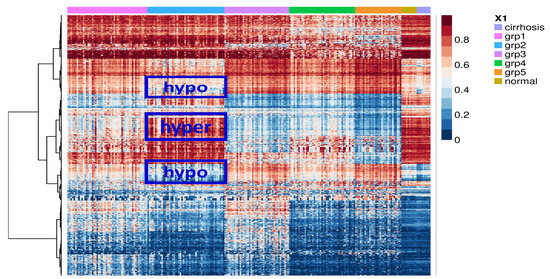
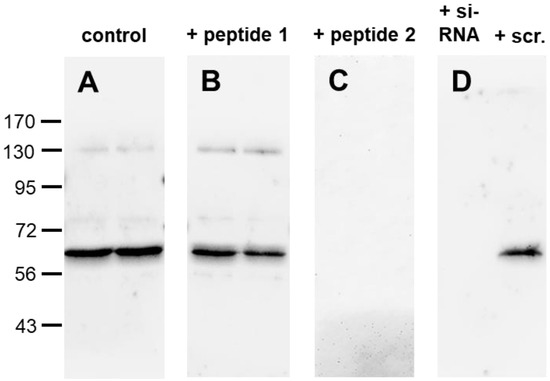
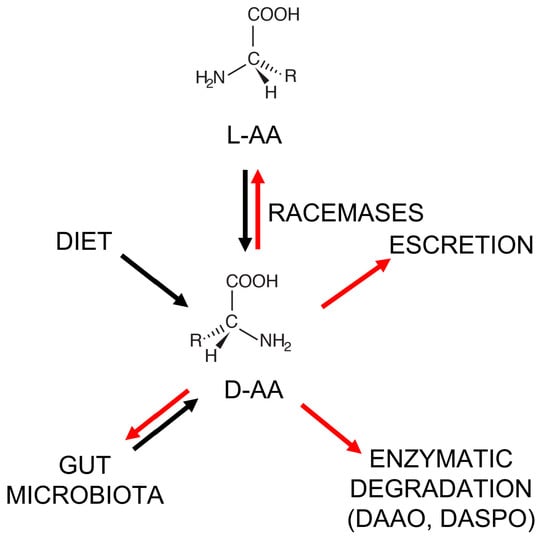
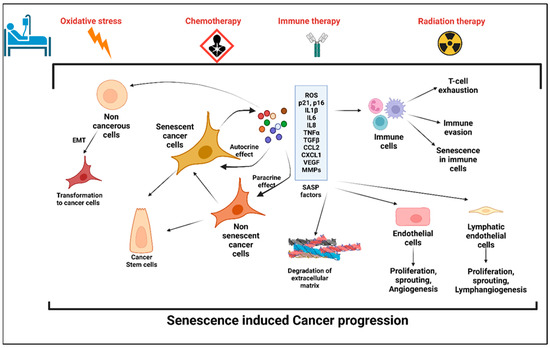
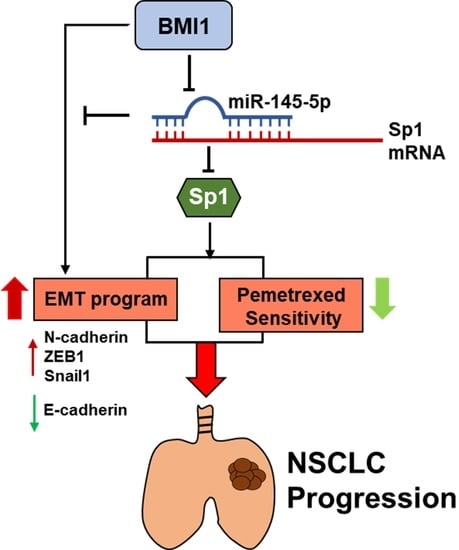
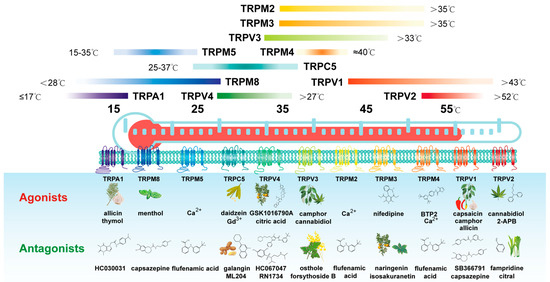
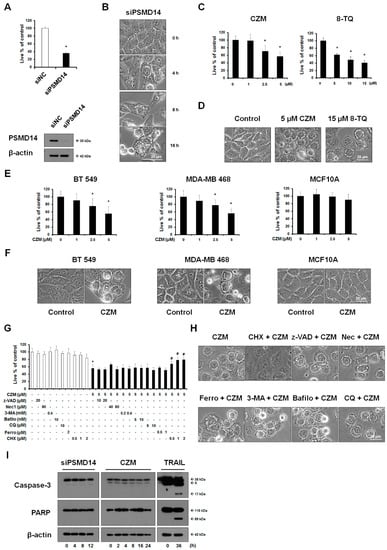
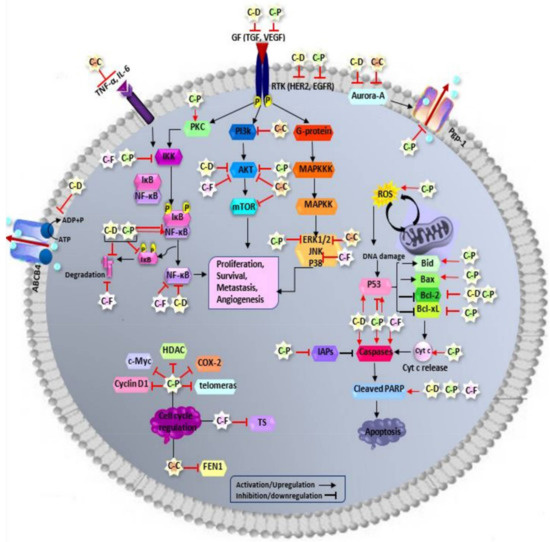
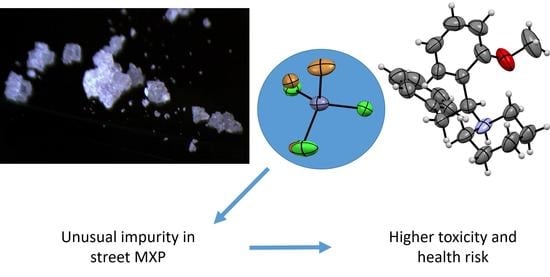
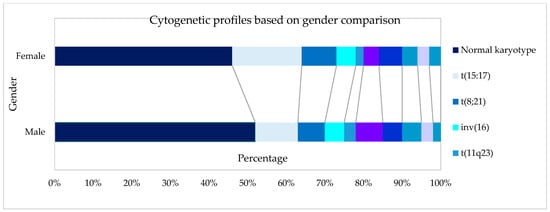
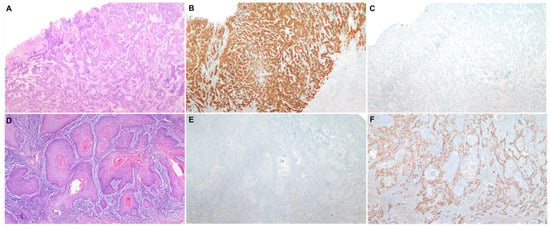
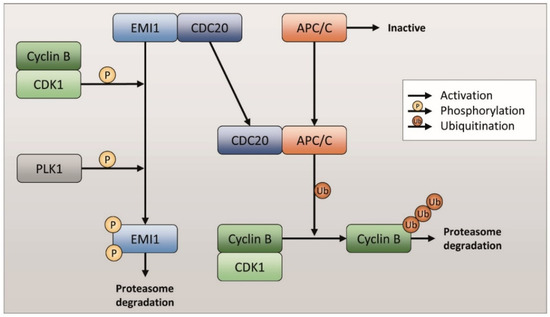
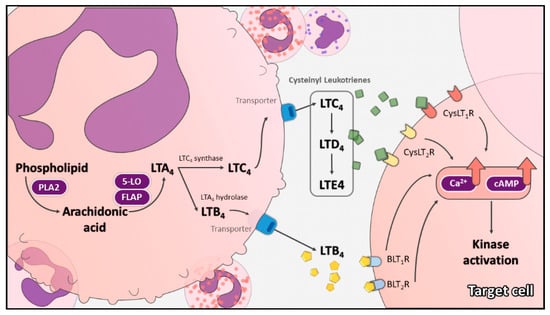
Feature Papers in Molecular Oncology
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Oncology".
Viewed by 370700Editor
2. Cancer Health Equity Institute, Morehouse School of Medicine, Atlanta, GA 30310, USA
Interests: cancer progression and metastasis; tumor immunobiology; cancer health disparity
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
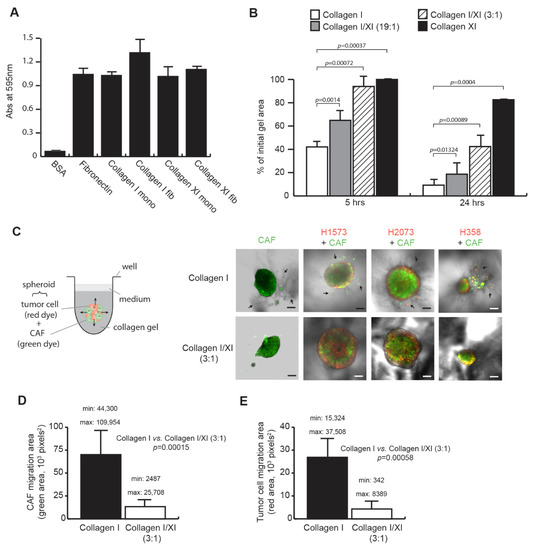
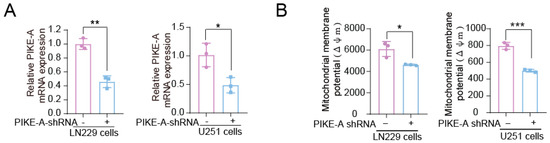
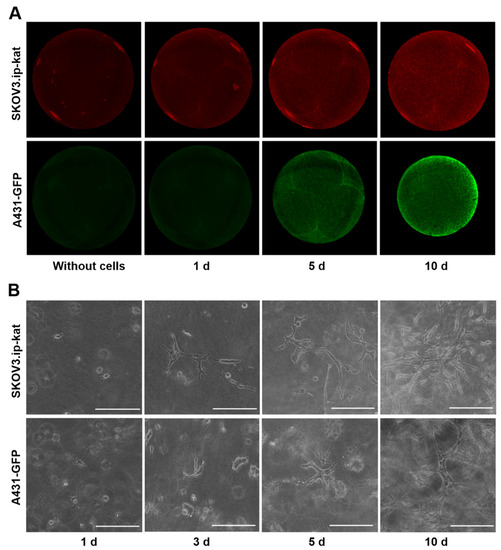
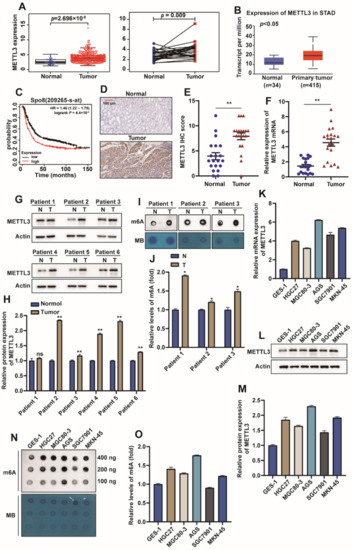
The special edition “Feature Papers in Molecular Oncology” will comprise important contributions by scholars in the field of cancer biology and Editorial Board members of the IJMS section Molecular Oncology. Their broad expertise will result in a comprehensive array of the latest findings in this field, and we thus encourage submissions of high-quality research articles or reviews. Suitable topics for this special edition include cancer genomics, transcriptomics, proteomics and metabolomics, as well as basic molecular and cell biological mechanisms of apoptosis, the cell cycle, cell–cell interactions, cell migration, and exosomes. The papers on basic molecular mechanisms will be interwoven with studies on the complexity of the cancer niche responsible for promoting cancer expansion and metastasis. Niche components of relevance comprise, among others, the vasculature, immune environment, and fibroblasts, and their related effects on EMT, cell proliferation, cancer cell invasion, and seeding at distant sites, as well as regulating mechanisms suppressing tumor cell expansion. Comprehensive studies of cancer must carry a translational component: for this, bioinformatics is essential. Finally, studies addressing early detection by imaging, biomarkers, and pathology together with novel treatment regimens are also appropriate. We look forward to your submissions to this anticipated collection of exciting, top-quality papers covering the latest findings in the above listed research areas of molecular oncology.
Prof. Dr. Shailesh Singh
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.